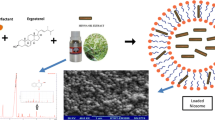
Abstract
Background
The use of phytochemicals to prevent or suppress tumours is known as chemoprevention. Numerous plant-derived agents have been reported to have anticancer potentials. As one such anticancer phytochemical, diosgenin has several applications which are nevertheless limited due to its low solubility in water.
Methods
We loaded diosgenin into niosome to increase its solubility and hence efficiency. Diosgenin-niosome (diosgenin loaded into niosome) was prepared by thin-film hydration method and characterised by optical microscopy, dynamic light scattering (DLS), scanning electron microscopy (SEM), and UV-visible spectrophotometry. Also, loading efficiency, in vitro drug release, and cytotoxicity assay were performed on HepG2 cell line.
Results and discussion
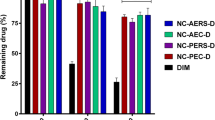
Diosgenin-niosome has a nanometric size with a normal size distribution and spherical morphology. The loading efficiency of diosgenin was about 89% with a sustainable and controllable release rate. Finally, the viability of free diosgenin was 61.25%, and after loading into niosomes, it was improved to 28.32%.
Conclusion
The results demonstrated that niosomes increase the solubility of naturally derived hydrophobic chemicals and thus enhance their anticancer effect.

Graphical abstract









Similar content being viewed by others
References
Radhi WA, Ismael SM, Al-Shawi JM, Hussein KA. Quantitative structure–activity relationship studies of flavonoids substituted as anticancer agents activity against the growth of the hepatic Cancer cell lines HepG2. Int J Chem. 2017;9(2):1.
Wang W-W, Wang Y-B, Wang D-Q, Lin Z, Sun R-J. Integrin beta-8 (ITGB8) silencing reverses gefitinib resistance of human hepatic cancer HepG2/G cell line. Int J Clin Exp Med. 2015;8(2):3063–71.
Chow EKH, Ll F, Chen X, Bishop JM. Oncogene-specific formation of chemoresistant murine hepatic cancer stem cells. Hepatology. 2012;56(4):1331–41.
Singh A, Dar MY, Joshi B, Sharma B, Shrivastava S, Shukla S. Phytofabrication of silver nanoparticles: novel drug to overcome hepatocellular ailments. Toxicol Rep. 2018;5:333–42.
Yue M, Shen X, Liu Y, Lin X, Zhou S, Song Y. Effect of wilfortrine on human hepatic cancer HepG2 cell proliferation potential in vitro. Genet Mol Res. 2015;14(4):15349–55.
Li WJ, Lian YW, Guan QS, Li N, Liang WJ, Liu WX, et al. Liver-targeted delivery of liposome-encapsulated curcumol using galactosylated-stearate. Exp Ther Med. 2018;16(2):925–30.
Selim S, Al Jaouni S. Anticancer and apoptotic effects on cell proliferation of diosgenin isolated from Costus speciosus (Koen.) Sm. BMC Complement Altern Med. 2015;15(1):301.
Marrelli M, Cristaldi B, Menichini F, Conforti F. Inhibitory effects of wild dietary plants on lipid peroxidation and on the proliferation of human cancer cells. Food Chem Toxicol. 2015;86:16–24.
Marzouk M, Soliman A, Omar T. Hypoglycemic and antioxidative effects of fenugreek and termis seeds powder in streptozotocin-diabetic rats. Eur Rev Med Pharmacol Sci. 2013;17(4):559–65.
Yan L, Zhang Y, Gao W, Man S, Wang Y. In vitro and in vivo anticancer activity of steroid saponins of Paris polyphylla var. yunnanensis. Experimental Oncology. 2009;31(1):27–32.
Janicka K, Jastrzebska I, Petelska AD. The equilibria of Diosgenin–phosphatidylcholine and Diosgenin–cholesterol in monolayers at the air/water Interface. J Membr Biol. 2016;249(4):585–90.
Sethi G, Shanmugam MK, Warrier S, Merarchi M, Arfuso F, Kumar AP, et al. Pro-apoptotic and anti-Cancer properties of Diosgenin: a comprehensive and critical review. Nutrients. 2018;10(5):645.
Chen P-S, Shih Y-W, Huang H-C, Cheng H-W. Diosgenin, a steroidal saponin, inhibits migration and invasion of human prostate cancer PC-3 cells by reducing matrix metalloproteinases expression. PLoS One. 2011;6(5):e20164.
Lepage C, Léger D, Bertrand J, Martin F, Beneytout J, Liagre B. Diosgenin induces death receptor-5 through activation of p38 pathway and promotes TRAIL-induced apoptosis in colon cancer cells. Cancer Lett. 2011;301(2):193–202.
Léger DY, Liagre B, Cardot PJP, Beneytout J-L, Battu S. Diosgenin dose-dependent apoptosis and differentiation induction in human erythroleukemia cell line and sedimentation field-flow fractionation monitoring. Anal Biochem. 2004;335(2):267–78.
Das S, Dey KK, Dey G, Pal I, Majumder A, MaitiChoudhury S, et al. Antineoplastic and apoptotic potential of traditional medicines thymoquinone and diosgenin in squamous cell carcinoma. PLoS One. 2012;7(10):e46641.
Kim DS, Jeon BK, Lee YE, Woo WH, Mun YJ. Diosgenin induces apoptosis in HepG2 cells through generation of reactive oxygen species and mitochondrial pathway. Evid Based Complement Alternat Med. 2012;2012:1–8.
Li Y, Wang X, Cheng S, Du J, Deng Z, Zhang Y, et al. Diosgenin induces G2/M cell cycle arrest and apoptosis in human hepatocellular carcinoma cells. Oncol Rep. 2015;33(2):693–8.
Mao Z-J, Tang Q-J, Zhang C-A, Qin Z-F, Pang B. Wei P-k et al. anti-proliferation and anti-invasion effects of diosgenin on gastric cancer BGC-823 cells with HIF-1α shRNAs. Int J Mol Sci. 2012;13(5):6521–33.
Mohammad RY, Somayyeh G, Gholamreza H, Majid M, Yousef R. Diosgenin inhibits hTERT gene expression in the A549 lung cancer cell line. Asian Pac J Cancer Prev. 2013;14(11):6945–8.
Patel K, Gadewar M, Tahilyani V, Patel DK. A review on pharmacological and analytical aspects of diosgenin: a concise report. Nat Prod Bioprospect. 2012;2(2):46–52.
Ghosh S, More P, Derle A, Kitture R, Kale T, Gorain M, et al. Diosgenin functionalized iron oxide nanoparticles as novel nanomaterial against breast cancer. J Nanosci Nanotechnol. 2015;15(12):9464–72.
Sung B, Prasad S, Yadav VR, Aggarwal BB. Cancer cell signaling pathways targeted by spice-derived nutraceuticals. Nutr Cancer. 2012;64(2):173–97.
Raju J, Rao CV. Diosgenin, a steroid saponin constituent of yams and fenugreek: emerging evidence for applications in medicine. In: Bioactive compounds in Phytomedicine: InTech; 2012.
Jesus M, Martins AP, Gallardo E, Silvestre S. Diosgenin: recent highlights on pharmacology and analytical methodology. J Anal Methods Chem. 2016;2016:1–16.
Binesh A, Devaraj SN, Halagowder D. Atherogenic diet induced lipid accumulation induced NFκB level in heart, liver and brain of Wistar rat and diosgenin as an anti-inflammatory agent. Life Sci. 2018;196:28–37.
Uchegbu IF, Vyas SP. Non-ionic surfactant based vesicles (niosomes) in drug delivery. Int J Pharm. 1998;172(1–2):33–70.
Perchyonok V, Souza J, Zhang S, Moodley D, Grobler S. Bio-active designer materials and dentures: from design to application. Int J Med Nano Res. 2015;2:012.
Bonifácio BV, da Silva PB, dos Santos Ramos MA, Negri KMS, Bauab TM, Chorilli M. Nanotechnology-based drug delivery systems and herbal medicines: a review. Int J Nanomedicine. 2014;9:1.
Nie S. Understanding and overcoming major barriers in cancer nanomedicine. Nanomedicine. 2010;5(4):523–8.
Ebrahimi AK, Barani M, Sheikhshoaie I. Fabrication of a new superparamagnetic metal-organic framework with core-shell nanocomposite structures: characterization, biocompatibility, and drug release study. Mater Sci Eng C. 2018;92:349–55.
Davarpanah F, Yazdi AK, Barani M, Mirzaei M, Torkzadeh-Mahani M. Magnetic delivery of antitumor carboplatin by using PEGylated-Niosomes. DARU J Pharm Sci. 2018:1–8.
Banerjee S, Kundu A. Lipid-drug conjugates: a potential nanocarrier system for oral drug delivery applications. DARU J Pharm Sci. 2018;26(1):65–75.
Hassanzadeh P, Atyabi F, Dinarvand R, Dehpour A-R, Azhdarzadeh M, Dinarvand M. Application of nanostructured lipid carriers: the prolonged protective effects for sesamol in in vitro and in vivo models of ischemic stroke via activation of PI3K signalling pathway. DARU J Pharm Sci. 2017;25(1):25.
Chen C, Zheng H, Xu J, Shi X, Li F, Wang X. Sustained-release study on Exenatide loaded into mesoporous silica nanoparticles: in vitro characterization and in vivo evaluation. DARU J Pharm Sci. 2017;25(1):20.
Barani M, Nematollahi MH, Zaboli M, Mirzaei M, Torkzadeh-Mahani M, Pardakhty A, et al. In silico and in vitro study of magnetic niosomes for gene delivery: the effect of ergosterol and cholesterol. Mater Sci Eng C. 2018.
Rameshk M, Sharififar F, Mehrabani M, Pardakhty A, Farsinejad A, Mehrabani M. Proliferation and in vitro wound healing effects of the Microniosomes containing Narcissus tazetta L. bulb extract on primary human fibroblasts (HDFs). DARU J Pharm Sci. 2018;26(1):31–42.
Moghassemi S, Hadjizadeh A. Nano-niosomes as nanoscale drug delivery systems: an illustrated review. J Control Release. 2014;185:22–36.
Pathak S. Bioavailability Enhancement of Poorly Water-soluble Nano Diosgenin by Encapsulation using Chitosan/Bovine Serum Albumin Bilayers. Asian J Pharm (AJP). 2018;12:02.
Wei Z, Xin G, Wang H, Zheng H, Ji C, Gu J, et al. The diosgenin prodrug nanoparticles with pH-responsive as a drug delivery system uniquely prevents thrombosis without increased bleeding risk. Nanomedicine. 2018;14(3):673–84.
Basiri L, Rajabzadeh G, Bostan A. α-Tocopherol-loaded niosome prepared by heating method and its release behavior. Food Chem. 2017;221:620–8.
Addas R, Taboada P, Hajinezhad MR, Barani M, Beyzaei H. Effect of tocopherol on the properties of Pluronic F127 microemulsions: Physico-chemical characterization and in vivo toxicity. J Mol Liq. 2018.
Washington C. Drug release from microdisperse systems: a critical review. Int J Pharm. 1990;58(1):1–12.
Yeo L, Olusanya T, Chaw C, Elkordy A. Brief effect of a small hydrophobic drug (Cinnarizine) on the physicochemical characterisation of Niosomes produced by thin-film hydration and microfluidic methods. Pharmaceutics. 2018;10(4):185.
Hans M, Lowman A. Biodegradable nanoparticles for drug delivery and targeting. Curr Opinion Solid State Mater Sci. 2002;6(4):319–27.
Yang W, Hu Q, Xu Y, Liu H, Zhong L. Antibody fragment-conjugated gemcitabine and paclitaxel-based liposome for effective therapeutic efficacy in pancreatic cancer. Mater Sci Eng C. 2018;89:328–35.
Barani M, Mirzaei M, Torkzadeh-Mahani M, Nematollahi MH. Lawsone-loaded Niosome and its antitumor activity in MCF-7 breast Cancer cell line: a Nano-herbal treatment for Cancer. DARU J Pharm Sci. 2018;26(1):11–7.
Pando D, Caddeo C, Manconi M, Fadda AM, Pazos C. Nanodesign of olein vesicles for the topical delivery of the antioxidant resveratrol. J Pharm Pharmacol. 2013;65(8):1158–67.
Suriyaprakash TNK, Parthiban S, Prabu SL, Sumathi A. Formulation and evaluation of paclitaxel niosome for its improved anti-cancer activity. ACTA Pharm Sci. 2011;53(3).
Jain R, Sukla SK, Nema N, Panday A. Drug Nano-particle: a release kinetics. J Nanosci Nanotechnol. 2015;6(5):1.
Xu Y-Q, Chen W-R, Tsosie JK, Xie X, Li P, Wan J-B, et al. Niosome encapsulation of curcumin. J Nanomater. 2016;2016:15.
Han W, Wang S, Liang R, Wang L, Chen M, Li H, et al. Non-ionic surfactant vesicles simultaneously enhance antitumor activity and reduce the toxicity of cantharidin. Int J Nanomedicine. 2013;8:2187.
Acknowledgments
The authors are grateful to the Graduate University of Advanced Technology, Kerman, Iran and Rafsanjan University of Medical Sciences, Rafsanjan, Iran for their excellent technical and financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hajizadeh, M.R., Parvaz, N., Barani, M. et al. Diosgenin-loaded niosome as an effective phytochemical nanocarrier: physicochemical characterization, loading efficiency, and cytotoxicity assay. DARU J Pharm Sci 27, 329–339 (2019). https://doi.org/10.1007/s40199-019-00277-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40199-019-00277-0