Abstract
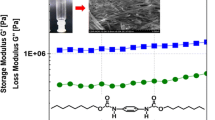
Fmoc or Boc mono-substituted cyclo(L-Lys-L-Lys)s were synthesized via the reaction of lysine cyclic dipeptide with Fmoc N-hydroxysuccinimide este(Fmoc-OSu) and di-tert-butyl dicarbonate[(Boc)2O], respectively. The resulted mono-substituted cyclo(L-Lys-L-Lys)s(2―4) by means of test tube inversion method served as organogelators enabled to form stable thermo-reversible organogels in alcoholic, substituted benzene and chlorinated solvents, with the minimum gelation concentration(MGC) in a range of 1%―4%(mass fraction). The transmission electron microscopy(TEM) and scanning electron microscopy(SEM) observations reveal that these gelators self-assembled into 3D nanofiber, nanoribbon or nanotube network structures. The rheological measurement exhibited that the storage modulus of gels is higher than the loss one, and the complex viscosity is reduced linearly with the increasing of scanning frequency. The fluorescence spectrum of compound 2 in 1,2-dichloroethane and benzene demonstrates that the emission peak of Fmoc at 320 nm has red-shifted and the intensity decreases gradually, while the intensity of the emission peak at 460 nm substantially enhances as a function of concentration, indicating the existence of π-π stacking interactions and the formation of J-type aggregates. Meanwhile, compound 4 self-assembled into nanotubes via the stacking of multiple bilayer membranes. Fmoc and Boc disubstituted cyclo(L-Lys-L-Lys)(3) holds the relatively lower MGC values, showing the stronger gelation ability in most selected organic solvents due to the presence of both Fmoc and Boc groups.
Similar content being viewed by others
References
Steed J. W., Chem. Commun., 2011, 47, 1379
Suzaki Y., Taira T., Osakada K., J. Mater. Chem., 2011, 21, 930
Hanabusa K., Suzuki M., Polym. J., 2014, 46, 776
Yang Z., Gu H., Zhang Y., Wang L., Xu B., Chem. Commun., 2004, 208
Zhang Y., Gu H., Yang Z., Xu B., J. Am. Chem. Soc., 2003, 125, 13680
Borthwick A. D., Chem. Rev., 2012, 112, 3641
Marchini M., Mingozzi M., Gennari C., Chem. Eur. J., 2012, 18, 6195
Dufour E., Garcia J., Org. Biomol. Chem., 2014, 12, 4964
Ou C. W., Wang H. M., Chen M. S., Chin. J. Chem., 2012, 30, 1781
Sasaki Y., Akustu Y., Suzuki K., Kisara K., Chem. Pharm. Bull., 1982, 30, 4435
Xie Z. G., Zhang A. Y., Ye L., Wang X., Feng Z. G., Soft Matter, 2009, 5, 1474
Hanabusa K., Fukui H., Suzuki M., Shirai H., Langmuir, 2005, 21, 10383
Hoshizawa H., Minemura Y., Yoshikawa K., Suzuki M., Hanabusa K., Langmuir, 2013, 29, 14666
Xie Z. G., Zhang A. Y., Ye L., Wang X., Feng Z. G., J. Mater. Chem., 2009, 19, 6100
Zong Q. Y., Geng H. M., Wang L., Ye L., Zhang A. Y., Feng Z. G., Acta Chim. Sinica, 2015, 73, 423
Geng H. M., Zong Q. Y., Ye L., Zhang A. Y., Feng Z. G., Chin. J. Appl. Chem., 2015, 32, 900
Geng H. M., Zong Q. Y., You J., Ye L., Zhang A. Y., Shao Z. Q., Feng Z. G., Sci. China Chem., 2015, 59, 293
Huang Z., Kang S. K., Banno M., Yamaguchi T., Lee D., Seok C., Yashima E., Science, 2012, 337, 1521
Eisele D. M., Cone C. W., Bloemsma E. A., Vlaming S. M., Rabe J. P., Vanden B. D., A. Nat. Chem., 2012, 4, 655
Zhang W., Jin W. S., Fukushima T., Saeki A., Seki S., Aida T., Science, 2011, 334, 340
Xie Z. G., Zhang A. Y., Ye L., Feng Z. G., Acta Chim. Sinica, 2008, 66, 2620
Maria A. M., Jordi J. B., Macromol. Chem. Phys., 2006, 207, 615
Kaur N., Zhou B., Breitbeil F., Hardy K., Trantcheva I., Mol. Pharm., 2008, 2, 294
Raeburn J., Cristina M. C., Adams D. J., Soft Matter, 2015, 11, 927
Lange S. C., Unsleber J., Wallera P. M., Ravoo B. J., Org. Biomol. Chem., 2015, 13 561
Huang R. L., Qi W., Feng L. B., Su R. X., He Z. M., Soft Matter, 2011, 7, 6222
Fichman G., Manohar S., Guterman T., Seliktar D., Messersmith P. B., Gazit E., NANO, 2014, 8, 7220
Zhang F. J., Xu Z. H., Dong S. L., Feng L., Song A. X., Soft Matter, 2014, 10, 4855
Qin S. Y., Wang Q. R., Peng M. Y., Zhang X. Z., Chin. J. Chem., 2014, 32, 22
Skilling K. J., Citossi F., Bradshaw T. D., Ashford M., Kellam B., Marlow M., Soft Matter, 2014, 10, 237
Yang Z. M., Xu B., J. Mater. Chem., 2007, 17, 2385
Solsona M. T., Miravet J. F., Chem. Eur. J., 2014, 20, 1023
Li J. L., Liu X. Y., Adv. Funct. Mater., 2010, 20, 3196
Shimizu T., Minamikawa H., Masuda M., Polym. J., 2014, 46, 831
Smith A. M., Williams R. J., Uljin R. V., Adv. Mater., 2008, 20, 37
Manchineella S., Govindaraju T., RSC Adv., 2012, 2, 5539
Kameta N., Minamikawa H., Masuda M., Soft Matter, 2011, 7, 4539
Masuda M., Shimizu T., Langmuir, 2004, 20, 5969
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Natural Science Foundation of China(No.21174018).
Rights and permissions
About this article
Cite this article
Zong, Q., Geng, H., Ye, L. et al. Synthesis and gelation capability of Fmoc and Boc mono-substituted cyclo(L-Lys-L-Lys)s. Chem. Res. Chin. Univ. 32, 484–492 (2016). https://doi.org/10.1007/s40242-016-5471-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40242-016-5471-5