Abstract
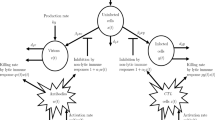
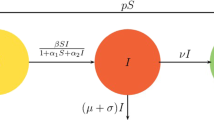
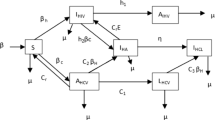
A vector-borne disease model with general incidence rates is proposed and investigated in this paper, where both vector and host are stratified by infection ages in the form of a hyperbolic system of partial differential equations coupled with ordinary differential equations. The existence, uniqueness, nonnegativeness, and boundedness of solution of the model are studied for biologically reasonable purpose. Furthermore, a global threshold dynamics of the system is established by constructing suitable Lyapunov functionals, which is determined by the basic reproduction number \(\mathcal {R}_0\): the infection-free equilibrium is globally asymptotically stable when \(\mathcal {R}_0<1\) while the endemic equilibrium is globally asymptotically stable when \(\mathcal {R}_0>1\).
Similar content being viewed by others
References
Altizer S, Dobson A, Hosseini P et al (2006) Seasonality and the dynamics of infectious diseases. Ecol Lett 9(4):467–484
Anderson RM, May RM (1978) Regulation and stability of host-parasite population interactions: I. Regulatory processes. J Anim Ecol 47:219–247
Antonovics J, Iwasa Y, Hassell MP (1995) A generalized model of parasitoid, venereal, and vector-based transmission processes. Am Nat 145(5):661–675
Avila-Vales E, Buonomo B (2015) Analysis of a mosquito-borne disease transmission model with vector stages and nonlinear forces of infection. Ricerche di Matematica. 64(2):377–390
Aron JL (1988) Mathematical modelling of immunity to malaria. Math Biosci 90(1):385–396
Barlow ND (2000) Non-linear transmission and simple models for bovine tuberculosis. J Anim Ecol 69(4):703–713
Begon M, Hazel SM, Baxby D et al (1999) Transmission dynamics of a zoonotic pathogen within and between wildlife host species. Proc R Soc Lond B Biol Sci 266(1432):1939–1945
Bhatt S, Gething PW, Brady OJ, Messina JP et al (2013) The global distribution and burden of dengue. Nature 496:504–507
Bowman C, Gumel AB, Van den Driessche P et al (2005) A mathematical model for assessing control strategies against West Nile virus. Bull Math Biol 67(5):1107–1133
Brand SPC, Rock KS, Keeling MJ (2016) The interaction between vector life history and short vector life in vector-borne disease transmission and control. PLoS Comput Biol 12(4):e1004837
Briggs CJ, Godfray HCJ (1995) The dynamics of insect-pathogen interactions in stage-structured populations. Am Nat 145(6):855–887
Browne CJ, Pilyugin SS (2013) Global analysis of age-structured within-host virus model. Discret Contin Dyn Syst Ser B 18(8):1999–2017
Capasso V, Grosso E, Serio G (1977) I modelli matematici nella indagine epidemiologica. Applicazione all’epidemia di colera verificatasi in Bari nel 1973 (italian). Annali Sclavo. 1977(19):193–208
Capasso V, Serio G (1978) A generalization of the Kermack-Mc Kendrick deterministic epidemic model. Math Biosci 42:41–61
Caminade C, McIntyre MK, Jones AE (2016) Climate change and vector-borne diseases: where are we next heading? J Infect Dis 214(9):1300–1301
Centers for Disease Control and Prevention (2002) Provisional surveillance summary of the West Nile virus epidemic-United States, January–November 2002. MMWR. Morbid Mortality Week Rep 51(50):1129–1133
Chen Y, Zou S, Yang J (2016) Global analysis of an SIR epidemic model with infection age and saturated incidence. Nonlinear Anal Real World Appl 30:16–31
Diekmann O, Kretzschmar M (1991) Patterns in the effects of infectious diseases on population growth. J Math Biol 29(6):539–570
Dietz K, Molineaux, L, Thomas A (1974) A malaria model tested in the African savannah. Bull World Health Org 50(3–4):347
Feng X, Ruan S, Teng Z, Wang K (2015) Stability and backward bifurcation in a malaria transmission model with applications to the control of malaria in China. Math Biosci 266:52–64
Feng Z, Velasco-Hernández JX (1997) Competitive exclusion in a vector-host model for the dengue fever. J Math Biol 35(5):523–544
Forouzannia F, Gumel AB (2014) Mathematical analysis of an age-structured model for malaria transmission dynamics. Math Biosci 247(2):80–94
Georgescu P, Hsieh YH (2006) Global stability for a virus dynamics model with nonlinear incidence of infection and removal. SIAM J Appl Math 67:337–353
Hale JK (1988) Asymptotic behavior of dissipative systems. Am. Math. Soc, Providence, RI
Hethcote HW (2000) The mathematics of infectious diseases. SIAM Rev 42(4):599–653
Hirsch WM, Hanisch H, Gabriel J-P (1985) Differential equation models of some parasitic infections: methods for the study of asymptotic behavior. Commun Pure Appl Math 38:733–753
Hochberg ME (1991) Non-linear transmission rates and the dynamics of infectious disease. J Theoret Biol 153(3):301–321
Hollingsworth TD, Pulliam JRC, Funk S et al (2015) Seven challenges for modelling indirect transmission: vector-borne diseases, macroparasites and neglected tropical diseases. Epidemics 10:16–20
Iannelli M (1995) Mathematical theory of age-structured population dynamics. In: Applied mathematics monographs, vol 7, comitato Nazionale per le Scienze Matematiche, Consiglio Nazionale delle Ricerche (C.N.R.), Giardini, Pisa
Inaba H, Sekine H (2004) A mathematical model for Chagas disease with infection-age-dependent infectivity. Math Biosci 190(1):39–69
Knell RJ, Begon M, Thompson DJ (1996) Transmission dynamics of Bacillus thuringiensis infecting Plodia interpunctella: a test of the mass action assumption with an insect pathogen. Proc R Soc Lond B Biol Sci 263(1366):75–81
Korobeinikov A (2009) Global asymptotic properties of virus dynamics models with dose-dependent parasite reproduction and virulence and nonlinear incidence rate. Math Med Biol 26:225–239
Korobeinikov A (2007) Global properties of infectious disease models with nonlinear incidence. Bull Math Biol 69:1871–1886
Kuniya T (2014) Existence of a nontrivial periodic solution in an age-structured SIR epidemic model with time periodic coefficients. Appl Math Lett 27:15–20
Kuniya T, Oizumi R (2015) Existence result for an age-structured SIS epidemic model with spatial diffusion. Nonlinear Anal Real World Appl 23:196–208
Lutambi AM, Penny MA, Smith T, Chitnis N (2013) Mathematical modelling of mosquito dispersal in a heterogeneous environment. Math Biosci 241:198–216
Lashari AA, Zaman G (2011) Global dynamics of vector-borne diseases with horizontal transmission in host population. Comput Math Appl 61(4):745–754
Liu H, Yu J, Zhu G (2006) Analysis of a vector-host malaria model with impulsive effect and infection-age. Adv Comp Syst 9(3):237–248
Mandal S, Sarkar RR, Sinha S (2011) Mathematical models of malaria-a review. Malaria J 10(1):202
Macdonald G (1952) The analysis of equilibrium in malaria. Trop Dis Bull 49(9):813–829
Maidana NA, Yang HM (2008) Describing the geographic spread of dengue disease by traveling waves. Math Biosci 215(1):64–77
Magal P, McCluskey CC, Webb GF (2010) Lyapunov functional and global asymptotic stability for an infection-age model. Appl Anal 89(7):1109–1140
Anderson RM, May RM (1978) Regulation and stability of host-parasite population interactions: II. Destabilizing processes. J Anim Ecol 47:249–267
McCallum H, Barlow N, Hone J (2001) How should pathogen transmission be modelled? Trends Ecol Evol 16(6):295–300
Melnik AV, Korobeinikov A (2013) Lyapunov functions and global stability for SIR and SEIR models with age-dependent susceptibility. Math Biosci Eng 10(2):369–378
Nash D, Mostashari F, Fine A et al (2001) The outbreak of West Nile virus infection in the New York City area in 1999. N Engl J Med 344(24):1807–1814
Ngwa GA, Shu WS (2000) A mathematical model for endemic malaria with variable human and mosquito populations. Math Comput Model 32:747–763
Novoseltsev VN, Michalski AI, Novoseltseva JA et al (2012) An age-structured extension to the vectorial capacity model. PloS One 7(6):e39479
Park TR (2004) Age-dependence in epidemic models of vector-borne infections. The University of Alabama in Huntsville, Thesis
Qiu Z (2008) Dynamical behavior of a vector-host epidemic model with demographic structure. Comput Math Appl 56(12):3118–3129
Rock KS, Wood DA, Keeling MJ (2015) Age-and bite-structured models for vector-borne diseases. Epidemics 12:20–29
Roop-O P, Chinviriyasit W, Chinviriyasit S (2015) The effect of incidence function in backward bifurcation for malaria model with temporary immunity. Math Biosci 265:47–64
Ross R (1910) The prevention of malaria. J. Murray, London
Ross R (1911) Some quantitative studies in epidemiology. Nature 87:466–467
Ruan S, Xiao D, Beier JC (2008) On the delayed Ross-Macdonald model for malaria transmission. Bull Math Biol 70(4):1098–1114
Smith HL, Thieme HR (2011) Dynamical systems and population persistence. American Mathematical Society, Providence, RI
Tahir MT (2017) Incidence of dengue haemorrhagic fever in local population of Lahore. Pak Biomed 25(2):93–96
Thieme HR (2000) Uniform persistence and permanence for non-autonomous semiflows in population biology. Math Biosci 166:173–201
Tumwiine J, Mugisha JYT, Luboobi LS (2007) A mathematical model for the dynamics of malaria in a human host and mosquito vector with temporary immunity. Appl Math Comput 189(2):1953–1965
Saul A (1996) Transmission dynamics of Plasmodium falciparum. Parasitol Today 12:74–79
Soufiane B, Touaoula TM (2016) Global analysis of an infection age model with a class of nonlinear incidence rates. J Math Anal Appl 434:1211–1239
Vargas-De-León C (2012) Global analysis of a delayed vector-bias model for malaria transmission with incubation period in mosquitoes. Math Biosci Eng 9(1):165–174
Vargas-De-León C, Esteva L, Korobeinikov A (2014) Age-dependency in host-vector models: the global analysis. Appl Math Comput 243:969–981
Wang X, Lou Y, Song X (2017a) Age-structured within-host hiv dynamics with multiple target cells. Stud Appl Math 138(1):43–76
Wang X, Chen, Y, Liu S (2017b) Dynamics of an age-structured host-vector model for malaria transmission. Math Methods Appl Sci. https://doi.org/10.1002/mma.4723
Wang X, Chen Y, Martcheva M, Rong L (2017c) Threshold dynamics of an age-structured vector-borne disease model with general nonlinear incidence rates. Nonlinear Anal Real World Appl (Submitted)
Wang J, Guo M, Liu S (2017d) SVIR epidemic model with age structure in susceptibility, vaccination effects and relapse. IMA J Appl Math 82(5):945–970
Wang J, Zhang R, Kuniya T (2015) The dynamics of an SVIR epidemiological model with infection age. IMA J Appl Math 81(2):321–343
World Health Organization: (2016) Fact sheet: world malaria report 2016. http://www.who.int/malaria/media/world-malaria-report-2016/en/. Accessed Dec 2016
Yang J, Chen Y, Kuniya T (2017) Threshold dynamics of an age-structured epidemic model with relapse and nonlinear incidence. IMA J Appl Math 82(3):629–655
Acknowledgements
X. Wang is supported by NSFC (no. 11771374), the CSC (201508410281), the Nanhu Scholar Program for Young Scholars of Xinyang Normal University, the Program for Science and Technology Innovation Talents in Universities of Henan Province (17HASTIT011), the Universities Young Teachers Program of Henan Province (2014GGJS-093). Y. Chen is supported by NSERC. S. Liu is supported by NSFC (no. 11471089).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Geraldo Diniz.
Rights and permissions
About this article
Cite this article
Wang, X., Chen, Y. & Liu, S. Global dynamics of a vector-borne disease model with infection ages and general incidence rates. Comp. Appl. Math. 37, 4055–4080 (2018). https://doi.org/10.1007/s40314-017-0560-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40314-017-0560-8
Keywords
- Vector-borne disease model
- Infection age
- General incidence rate
- Uniform persistence
- Fluctuation lemma
- Lyapunov functional