Abstract
A subbituminous coal was oxidized with air at 150 °C on a fixed bed for 4 h and xanthated with carbon disulfide in a basic solution, at 30 or 5–10 °C. This xanthated coal was evaluated for the removal of Hg2+ and Cd2+ from 7,000 mg/L aqueous solutions; metal concentrations were determined by atomic absorption spectrometry. The ion exchange of the xanthated coal was compared against those of the original subbituminous coal, a sulfonated subbituminous coal, activated carbon, commercial activated carbon, and commercial synthetic resin. The commercial synthetic resin showed the highest exchange capacity (concentration factor 98 %) followed by the xanthated coal (concentration factor 96 %). The retention of cadmium on the sulfonated subbituminous coal was lower (exchange capacity 0.56 meq/g) than that of xanthated coals (1.85 ± 0.09 meq/g). Our xanthated coal showed a better Cd2+ removal (81 % against 15 %) than a non preoxidized 40-h-xanthated coal, which shows that oxidation of coal increased the amount of oxygenated groups which enhanced xanthation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The worldwide production of hard coal totaled over 6,185 million tonnes and low rank coal, such as lignite and brown coal, 1,042 million tonnes for 2012; of this hard coal, the production of Colombia is about 82 million tonnes. Most of this coal is exported for the generation of electricity, steel production, cement manufacturing, and as a liquid fuel (World Coal Association 2014). Combustion of coal generates environmental problems including carbon dioxide emission and fly ash production (Sun et al. 2009). Combustion of low rank coals generates more environmental problems than that of hard coals due to their low calorific capacity and high content of heteroatoms which introduce acids into the atmosphere during combustion; acids in air are responsible for the destruction of monuments and buildings, damage to rivers and lakes, forests and vegetation, terrestrial ecosystems and human health, and decrease of visibility (Jain et al. 2012); these problems have promoted research on the alternative use of low rank coal such as cheap ion exchangers which can be used for demineralization of water, removal of heavy metals from waste solutions and recovery of valuable metals.
Low rank coals, such as lignite and subbituminous coals, have natural ion-exchange properties because of carboxylic and phenolic groups attached to a highly cross-linked aromatic structure (Hayashi and Li 2004) and continues attracting scientific interest. Anwar et al. (2009) investigated the adsorption of Cr(III) by two coal varieties from Lakhra and Thar coalfields (Pakistan) as a function of adsorbent dose, pH, contact time and agitation speed; Cr(III) removal was 2.6 mg of Cr(III) per gram of Lakhra coal. In other study, the removal of Cr(VI) from aqueous solutions using low-rank Turkish brown coals was studied as a function of contact time, solution pH, temperature, concentration of metal solutions and amount of adsorbent; adsorption capacities of 12.4 mM of Cr(VI)/g were observed (Arslan and Pehlivan 2007). Rao et al. (2011) used lignite to remove Pb2+ from aqueous solution and 98 % adsorption of Pb2+ was achieved.
The exchange capacity of low rank coal can be increased by enhancing the content of functional groups through sulfonation (Martyniuk and Augustyn 1991), xanthation (Marañón and Sastre 1992) and other reactions. Orjuela et al. (2000) prepared sulfonated exchangers using four subbituminous coals from Colombia. Xanthation increases the coal functional groups content for complexing metals. The xanthate group bonds metals through free electrons in the CS2 group (Haenel 1992). Marañón and Sastre (1992) obtained cation exchangers by xanthation of apple pulp, a residual biomass coming from the cider production, which is formed by biopolymers such as cellulose and lignin; xanthation of apple pulp enlarged its structural stability and performance as ion exchanger.
In coal oxidation, there are chemical reactions between the oxidation agent and the functional groups of coal increasing carboxyl groups and oxygen content (Shi et al. 2012), required for reaction with xanthated groups. In this work, we studied the influence of oxidation in the properties of a cation exchanger obtained by xanthation of subbituminous coals from Montelíbano, Córdoba (Colombia), and used the exchanger to remove cadmium and mercury from aqueous solutions. The influence of xanthation temperature, coal particle size, and pre-oxidation with air on the exchange capacity of Cd2+ and Hg2+ were studied and the exchange capacity was compared against those of the original subbituminous coal, a sulfonated subbituminous coal, activated carbon, commercial activated carbon, and commercial synthetic resin. These studies are important in Colombia where low rank coal is almost totally used for highly contaminant activities such as energy and metal production.
2 Method
2.1 Instruments, materials and reagents
For the extraction and swelling experiments, analytical grade tetrahydrofuran was used; commercial grade NaOH and CS2 were used for xanthation. Two 7,000 ± 5 mg/L solutions of cadmium and mercury were prepared from analytical reagents [CdCl2 and Hg(NO3)2, Merck] for the exchange experiments. The exchange materials were a subbituminous coal from Montelíbano (Colombia), lab-prepared activated and sulfonated coals, a commercial synthetic resin and a commercial activated coal; calorific capacity (9,925 BTU Lb−1) and proximate and ultimate analysis were obtained in SGS (Barranquilla, Colombia) according to ASTM norms for coal analysis (Table 1).
An activated coal used was prepared in our laboratory from sugar cane bagasse with a surface area of 369 m2/g and acidity of 1.68 meq/g (Colpas-Castillo et al. 2011); the commercial synthetic resin was a Dowex Marathon (Dow Chemical) with sulfonic acid quelating groups (Lenntech 2014); the commercial activated coal was an specialty grade granular material produced by steam activation of lignite coal (Darco MRX 0.60–2.00 mm particle size, BET surface = 613 m2/g, total pore volume 0.62 cm3/g, acid washed). A Perkin Elmer Analyst 300 instrument was used for the flame and cold vapor atomic absorption measurements. Infrared spectra were taken in a FTIR Nicolet 5DXC instrument.
2.2 Sample preparation
The samples were stored in polyethylene bags after mining, ground in porcelain mortars, sieved to particle sizes of 0.1–2.5 mm, demineralized with HCl 0.5 N for 6 h, rinsed with distilled water until the pH of the resulting rinsing water was >6, and dried at 105 °C for 2 h.
Three coal groups with particle sizes of 0.1–0.5 (T1), 0.5–1.0 (T2), 1.0–2.5 (T3) mm were obtained. Samples were extracted and swollen with tetrahydrofuran during 3 days, washed with distilled water, and dried at 105 °C for 2 h. pH was adjusted using a carbonate–bicarbonate buffer solution.
2.3 First stage
In these experiments, the optimal conditions for xanthation were determined and sulfonation was performed.
2.3.1 Xanthation
Duplicate experiments were carried out for every coal particle size and reaction temperature. A 6 M NaOH solution was added to the coal and, after 5 min, CS2 in a 1:1 molar ratio with respect to NaOH, and 3:1 to the amount of OH in the coal, calculated from elemental analysis making coal oxygen content = coal OH content. Then, the mixture was magnetically stirred during 40 h at 5–10 or 30 °C.
2.3.2 Sulfonation
Sulfuric acid (98 %) was used at 150 °C during 75 min at a 1:4 ratio between acid and the T1 size coal. Then, the coal was washed up to pH > 6 and dried at 105 °C for 24 h.
2.3.3 Exchange capacity of xanthated and sulfonated coals
The exchange capacity of xanthated and sulfonated coals was determined as follows: For every combination of particle size and reaction temperature, 50.00 mL of 7,000 mg/L solutions of Cd2+ were mixed with 0.5000 g of xanthated or sulfonated coal by triplicate. After stirring for 24 h, the filtrate and eluate were analyzed by flame atomic absorption spectrometry.
2.4 Second stage
Based on the first stage experiments, T1 size coal was oxidized with air at 150 °C on a fixed bed for 4 h, xanthated following the procedure used in the first stage but at room temperature during 8 h, and stirred during 4 h. Then, the coal was washed to pH 7 and dried at 50 °C for 20 h.
3 Exchange experiments
50.00 mL of Hg2+ and Cd2+ solutions of 0.100, 1.000, 12.48, 124.8, and 998.3 mg/L were stirred with 0.5000 g of oxidized-xanthated coal, subbituminous coal, activated coal, commercial synthetic resin and commercial activated carbon at pH 8. After 6 h of stirring, the exchangers were filtered and eluted with HCl 0.5 N until 50.00 mL of eluate were completed. The eluted solutions were analyzed by flame atomic absorption spectrometry (for Cd2+) and cold vapor (for Hg2+).
The cation exchange capacity was expressed as a concentration factor, CF (Fehrmann and Pohl 1993), defined as the ratio of adsorbed mass of metal (in µg) per gram of exchanger to the cationic solution concentration using the formula:
where, V i the volume of the initial solution (mL), V f volume of the filtrate (mL), M sample mass (g), C i metallic concentration in the initial solution (mg/L), C f metallic concentration in the filtrate (mg/L). CF was used to show the onset of saturation of the exchangers with the increase of exchange solution concentration.
4 Results and discussion
We prepared a cation exchanger by xanthation of a subbituminous coal to remove Cd2+ and Hg2+ from aqueous solutions, studied the effect of temperature, particle size, and air oxidation on the exchange capacity and compared this exchanger to others we prepared or were commercially available.
4.1 Particle size, xanthation temperature and the exchange capacity
The infrared spectra of coal xanthated at 5–10 °C (Fig. 1) shows a CS2 band near 1,400 cm−1 slightly stronger than that of the coal xanthated at 30 °C; this indicates that at low temperature more CS2 groups were bonded to the coal; also, a slightly weaker OH phenolic stretching at 3,184 cm−1 indicates that more OH groups reacted at low temperature. This behavior can be attributed to a greater yield of reaction at low temperature because carbon disulfide is very volatile at 30 °C and its volatilization diminished the reagent required to complete xanthation. The exchange capacity (Table 2) shown by the coal xanthated at 5–10 °C (13 ± 2 for all particle sizes) was higher than that shown by the coal xanthated at 30 °C (12 ± 1 for all particle sizes) although there was not a significant difference (p > 0.05) (Fig. 2).
In the first stage of the experiments, the highest concentration factor for cadmium (15.0) was obtained for the T1 size coal xanthated at 5–10 °C followed by the T1 size coal xanthated at 30 °C (13.5). The smaller the particle size the greater the ability of the coals to remove cadmium due to the increase in surface area obtained with smaller particles which expose more xanthate groups for complexing metals; there was a significant difference (p < 0.05) for the exchange capacities between T1 size coal and both T2 and T3 but there was not between xanthation temperatures or T2 and T3.
4.2 Comparison between xanthated and sulfonated coal
The retention of cadmium on the sulfonated subbituminous coal was low (exchange capacity 0.56 meq/g) for a T1 size coal and had a significant difference with the cadmium exchange capacities of xanthated coals (1.85 ± 0.09 meq/g) (p < 0.05).
4.3 Comparison of the exchange capacity between a preoxidized xanthated coal and other exchangers for cadmium and mercury
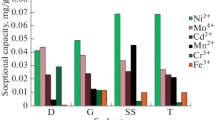
The performance of a preoxidized xanthated coal (XC) was compared with those of a commercial activated carbon (CA), an activated carbon prepared from our coal (AC), a commercial synthetic resin (CR) and the Montelíbano subbituminous coal without treatment (SC). The exchange capacities for each exchanger are shown in Fig. 2 and Table 3 for mercury and cadmium, and are expressed as CFs.
The graphs of CF against metallic solution concentration show a similar behavior for all the exchangers: the average CFs of all exchangers decreased with the increase of the exchange solution concentrations; CFs were 98.7 ± 0.9, 97 ± 4, 83 ± 19, 58 ± 37, 38 ± 43 for 50.00 mL solutions of 0.100, 1.000, 12.48, 124.8, and 998.4 mg/L of both metals, respectively. Moreover, the saturation capacity was reached with the solutions of higher metallic concentrations. The saturation of 0.5000 g of CA, SC and AC was practically reached with 50.00 mL solution of 998.4 mg/L for both metals.
In general, CFs shown by the exchangers were higher, although very close, for cadmium than for mercury, maybe because of the small ionic size of Cd2+ that allowed the metal to reach the smallest pores of the coal (Table 3). Both activated carbons and the non-treated subbituminous coal showed lower capacities to remove heavy metals from aqueous solutions than the commercial resin and xanthated coal; this is exemplified at 998.4 mg/L concentrations of exchange solutions for both metals where the activated carbons and non-treated subbituminous coal showed an average CF of 4 ± 1 and the commercial resin and xanthated coal an average CF of 88 ± 7; in these conditions, the commercial synthetic resin showed the highest exchange capacity (CF 98 ± 2) followed by our xanthated coal (CF 95.7 ± 0.1).
The preoxidized 8-h-xanthated coal prepared in this work showed a better Cd2+ removal (81 % against 15 %) than a non-preoxidized 40-h-xanthated coal, which suggests that the oxidation of coal increases the amount of oxygenated functional groups in the coal structure; this oxygenation enhance the extent of xanthation and increase the density of exchangers in the coal surface. This also implies that it is more important a previous oxidation to increase the oxygenated groups than a long xanthation time, for a better coal exchange capacity.
5 Conclusions
We oxidized and xanthated a subbituminous coal from Montelíbano Córdoba (Colombia) with carbon disulfide in a basic solution, at 30 or 5–10 °C at different coal particle sizes. The exchange capacity of the xanthated coal increased when the particle size decreased. Only a commercial synthetic resin showed a slightly higher exchange capacity than our xanthated coal. In general, Hg2+ was removed less effectively from the aqueous solutions than Cd2+. Oxidation, previous to xanthation, increased metal retention in the coal to values comparable with those of a commercial synthetic resin. In general, the exchange capacities for the exchangers followed this order: Commercial activated carbons < non-treated subbituminous coal < our activated carbon < sulfonated subbituminous coal < our xanthated coal < commercial synthetic resin.
References
Anwar J, Shafique U, Salman M, Zaman W, Anwar S, Anzano JM (2009) Removal of chromium(III) by using coal as adsorbent. J Hazard Mater 171(1–3):797–801
Arslan G, Pehlivan E (2007) Batch removal of chromium(VI) from aqueous solution by Turkish brown coals. Bioresour Technol 98(15):2836–2845
Colpas-Castillo F, Meza E, Fernandez-Maestre R, Primera-Pedrozo OM (2011) Carbones activados a partir de bagazo de caña de azúcar y zuro de maíz para la adsorción de cadmio y plomo. Rev Acad Colomb Cienc Exact, Fís Nat 35(136):303–312
Fehrmann C, Pohl P (1993) Cadmium adsorption by the non-living biomass of microalgae grown in axenic mass culture. J Appl Phycol 5(6):555–562
Haenel M (1992) Recent progress in coal structure research. Fuel 71(11):1211–1223
Hayashi JI, Li CZ (2004) Structure and properties of victorian brown coal. In: Li CZ (ed) Advances in the science of victorian brown coal. Elsevier, Chicago, pp 11–84
Jain R, Urban L, Balbach H, Webb MD (2012) Contemporary issues in environmental assessment. In: Jain R, Urban L, Balbach H, Webb MD (eds) Handbook of environmental engineering assessment. Elsevier, Boston, pp 361–447
Lenntech (2014) Dowex-Marathon-C. http://www.Lenntech.Com/Data-Sheets/Dowex-Marathon-C.Pdf. Accessed 4 June 2014
Marañón E, Sastre H (1992) Preconcentration and removal of trace metals from water by apple waste. Bioresour Technol 40(1):73–76
Martyniuk H, Augustyn D (1991) Sorption of metals cations on sulfonated brown coals. Fuel 70(4):551–556
Orjuela A, Civetta N, Rivera J, Boyacá A, Díaz J (2000) Preparación de intercambiadores catiónicos a partir de carbón. Rev Colomb Quím 29(2):53–59
Rao RAK, Khan MA, Rehman F (2011) Batch and column studies for the removal of lead(II) ions from aqueous solution onto lignite. Adsorpt Sci Technol 29(1):83–98
Shi KY, Tao XX, Hong FF, He H, Ji YH, Li JL (2012) Mechanism of oxidation of low rank coal by nitric acid. J Coal Sci Eng (China) 18(4):396–399
Sun YZ, Fu KM, Zhu H, Zhu TL (2009) Silica–alumina molar ratio and some factors effect on the synthesis of zeolites from fly ash. J Coal Sci Eng (China) 15(4):430–433
World Coal Association (2014) http://www.worldcoal.org/coal/coal-mining/. Accessed 4 June 2014
Acknowledgments
The authors acknowledge S.G.S. de Colombia S.A., CABOT de Colombia, PETCO de Colombia and COLCLINKER S.A. for their support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Gomez, L.M., Colpas-Castillo, F. & Fernandez-Maestre, R. Cation exchange for mercury and cadmium of xanthated, sulfonated, activated and non-treated subbituminous coal, commercial activated carbon and commercial synthetic resin: effect of pre-oxidation on xanthation of subbituminous coal. Int J Coal Sci Technol 1, 235–240 (2014). https://doi.org/10.1007/s40789-014-0033-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40789-014-0033-2