Abstract
We report the development of a novel visible response BiVO4/TiO2(N2) nanotubes photoanode for photoelectrocatalytic applications. The nitrogen-treated TiO2 nanotube shows a high carrier concentration rate, thus resulting in a high efficient charge transportation and low electron–hole recombination in the TiO2–BiVO4. Therefore, the BiVO4/TiO2(N2) NTs photoanode enabled with a significantly enhanced photocurrent of 2.73 mA cm−2 (at 1 V vs. Ag/AgCl) and a degradation efficiency in the oxidation of dyes under visible light. Field emission scanning electron microscopy, X-ray diffractometry, energy-dispersive X-ray spectrometer, and UV–Vis absorption spectrum were conducted to characterize the photoanode and demonstrated the presence of both metal oxides as a junction composite.
Graphical Abstract
Visible-light response BiVO4/TiO2(N2) naontubes photoelectrode was fabricated for photoelectrochemical water splitting and organic degradation in this paper.

Similar content being viewed by others
1 Introduction
The extreme shortage of natural resources and severe environmental problems caused by burning fossil fuels are pressing global concerns. In the past decades, many efforts were made to explore alternate energy sources. Photoelectrocatalytic (PEC) technology is widely recognized as an alternative energy source because it provides a highly efficient and eco-friendly route to produce renewable energy, and it degrades organic pollutants by the direct use of sunlight [1–4]. It can be achieved using a semiconductor photoanode/liquid junction, which drives an oxidation reaction. Therefore, in most PEC cells, the overall performance is primarily determined by the photoanode. However, it is still a challenge to synthesize a photoanode material that is chemically stable and has reasonably high incident light-to-current conversion efficiency in the visible range.
In recent years, Bi3+-based complex oxides that could absorb visible light effectively and with the advantage of price beneficial have been produced as alternative energy materials [5–8]. BiVO4 is a promising high efficient photoanode and photocatalysis material, with advantages of small optical band gaps (2.4 eV) and high stability, and low conduction band edges that overcome traditional photoanode materials, such as ZnO, TiO2, WO3, and Fe2O3 [9–13]. However, BiVO4 has the shortages of poor carrier transport properties and a substantially less efficient physical photoconversion rate [8].
One approach for alleviating these limitations is to use another semiconductor as support material to form a heterojunction that not only facilitates carrier transport but also enhances light absorption. Among various semiconductors, TiO2 has been intensively studied as a promising photoanode because it is stable, cost-effective, and has a negative flat band potential (∼0.2 V vs. RHE) (RHE, reversible hydrogen electrode) [14–18]. Recently, Xie et al. [19] found an unusual spatial transfer of visibly excited high-energy electrons of BiVO4 to TiO2, which indicated enhanced photoactivity in the heterojunction of BiVO4/TiO2 nanoparticles. Li et al. [20] demonstrated that a proper facet contact between BiVO4 and TiO2 nanoparticles was the key to improving the photoactivity of BiVO4. Recently, we studied one-dimensional (1D) nanostructured TiO2 coupled with a BiVO4 heterojunction with straight channels for electron transportation that reduced carrier diffusion lengths and improved charge collection efficiencies [21]. However, TiO2 has an intrinsically low mobility that limits the enhancement of photoactivity of the BiVO4–TiO2 heterojunction. Therefore, increasing the carrier concentration and also the conductivity in TiO2 is crucial to constructing a BiVO4–TiO2 heterojunction for a high-performance PEC cell.
In this study, we pre-treated TiO2 nanotubes in the nitrogen gas (TiO2(N2) NTs) and then coupled them with BiVO4 to form a BiVO4/TiO2(N2) NTs heterojunction. We find that the photocurrent is increased by approximately 30 % compared to those obtained by previously reported BiVO4/TiO2 NTs heterojunction [21]. Our PEC experiments further demonstrate the improved performance in the degradation of dyes. These results are attributed to the high carrier concentration of TiO2 NTs after annealing in a non-oxidizing atmosphere, as observed by Mott–Schottky spectra. In this case, the defects presented in the TiO2(N2) NTs increase the charge transfer kinetics, along with the reduced recombination losses due to trap filling. Thus, the charge transport between BiVO4 and TiO2 is enhanced to produce a higher photoactivity. This heterojunction provides useful insight into the design and fabrication of BiVO4-based photoanodes for potentially cost-effective and highly efficient PEC applications in large-scale applications.
2 Experimental Procedures
2.1 Preparation of BiVO4/TiO2(N2) NTs Photoanodes
TiO2 NTs were prepared by a template method in which ZnO nanowires (NWs) were transformed during a liquid-phase deposition (LPD) process. ZnO NWs were synthesized on FTO glass (2 × 2 cm2) after a hydrothermal treatment [22]. Next, a LPD treatment was conducted by placing ZnO NW substrates in a mixed solution of 50 mm (NH4)2TiF6 and 150 mm H3BO3 for 20 min at 25 °C [23]. After the LPD treatment, the sample was further annealed at 500 °C for 2 h in nitrogen gas, and nitrogen-treated TiO2 NTs were obtained and marked as TiO2(N2) NTs. For the fabrication of the BiVO4/TiO2(N2) NTs photoanode, a yellow precursor solutions of 300 mM Bi(NO3)3 and 300 mM NH4VO3 in 2 M HNO3 were deposited on the TiO2 NTs by spin coating [24]. Finally, the samples were sintered at 450 °C for 2 h in room air and yielded a yellow BiVO4/TiO2(N2) NTs film. For the control, the TiO2 NTs annealed in room air were used to prepare the BiVO4/TiO2 NTs photoanodes and bare BiVO4/FTO photoanodes were also prepared using the same procedure without the TiO2 NTs substrate.
2.2 Structural Characterization
The morphologies of the samples were characterized using field emission scanning electron microscopy and a microscope equipped with an energy-dispersive X-ray spectrometer (EDX) (FEI, Sirion200) and TEM (JEM-2100F, JEOL, Japan). The crystalline phase of the samples was characterized by X-ray diffractometry (XRD) (AXS-8 Advance, Bruker, Germany). X-ray photoelectron spectroscopy (XPS) measurements were performed on an ESCALAB250 XPS measuring system with a Mg Kα X-ray source. Optical absorption measurements were conducted in a Lamda 750 UV–Vis–IR spectrophotometer using an integrating sphere.
2.3 Photoelectrochemical Measurements
The photo responses of the BiVO4/TiO2 NTs photoanode were conducted using a three-electrode system with the Ag/AgCl electrode as the reference, platinum foil as the auxiliary electrode, and the samples as the working electrode. The working electrode potential and current were controlled by an electrochemical workstation (CHI 660c, CH Instruments Inc., TX, USA). A 350-W Xe lamp was used as a simulated light source, without further description, and all experiments were conducted under visible light (light intensity, 100 mW cm−2). The electrolyte was a 0.1 M Na2SO4 solution. The linear sweep voltammograms (LSV) were conducted under chopped light irradiation. The scan rate for the linear sweep voltammetry was 10 mV s−1. Photoluminescence (PL) measurements were conducted using an OmniPL-LF325 system with a 325 nm laser at room temperature. The incident photon-to-charge conversion efficiency (IPCE) was measured by a system comprising a monochromator (Zolix, P.R. China), a 500-W xenon arc lamp, a calibrated silicon photodetector, and a power meter. Mott–Schottky (impedance) spectra were recorded in 0.2 M Na2SO4 without light at a frequency of 1 kHz and a scan rate of 10 mV s−1.
Intensity modulated photocurrent spectroscopy (IMPs) was determined using an electrochemical workstation (ZENNIUM, ZAHNER-elecktrik GmbH & Co. KG, Germany) equipped with a controlled intensity modulated photospectroscopy setup (CIMPS, PP211, ZAHNER-elecktrik GmbH & Co. KG, Germany) after a two-electrode configuration. A white light lamp (WLC02, ZAHNER-elecktrik GmbH & Co. KG, Germany) was used as the light source. The modulated light in the frequency range of 0.1 Hz–1 kHz superimposed on a steady dc light with an intensity of 60 mW cm−2 was also used as a light source.
2.4 Organics Compounds Degradation
The PEC degradation of the methylene blue (MB) experiment was conducted under the following conditions: visible light irradiation (100 mW cm−2), vigorous stirring, 1.0 V (vs. Ag/AgCl) of electric bias, pH 7, and 0.1 M sodium sulfate as the supporting electrolyte. Before degradation test, the nitrogen was bubbled to remove oxygen from the solution. The initial concentration of MB solution was 10 mg L−1 and the reaction solution was 20 mL during the experiment. The degradation rates of the dyes were analyzed with an UV–Vis spectrophotometer (UV2102 PCS, UNICO, Shanghai).
3 Results and Discussion
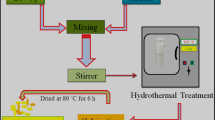
The main fabrication strategies for the BiVO4/TiO2(N2) NTs photoanodes are conducted in three steps as illustrated in Fig. 1. First, the ZnO NW template is grown on the FTO substrate through a hydrothermal method. Second, the template is transformed to TiO2 NTs after an LPD treatment which involves hydrolysis of ammonium hexafluorotitanate, and leads to the deposition of TiO2 as well as mild etching of ZnO from the formation of HF. Third, BiVO4 is deposited on the TiO2 NTs to form a photoactive composite layer.
Figure 2 shows the top and cross-sectional SEM images of optimized TiO2(N2) NTs and BiVO4/TiO2(N2) NTs, respectively. As shown in Fig. 2a, b, the obtained TiO2(N2) NTs have a vertical geometric shape, although the treatment of the NWs leads to partial connectivity among the constituent wires due to the surface tension during the evaporation of the solvent (Fig. 2a). Compared with the nitrogen-treated TiO2 NTs, the geometry for the air-annealed TiO2 NTs remains unchanged (not presented here). The TiO2 NTs are approximately 400 nm in length with a relatively rough surface (Fig. 2b). The top view SEM images of the BiVO4/TiO2(N2) NTs reveal that the TiO2(N2) NTs are completely covered by BiVO4 (Fig. 2c). Likewise, the side view also confirms the formation of the heterojunction of the BiVO4/TiO2(N2) NTs heterojunction (Fig. 2d). The thickness of the junction is approximately 600 nm, which is thicker than that of pure BiVO4 photoanode (Fig. S1). As shown in Fig. S2, the TEM images also demonstrate the heterojunction structure, where the BiVO4 nanoparticles are clearly observed on the TiO2 NTs.
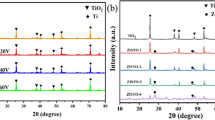
The elemental composition of the BiVO4/TiO2(N2) NTs was also analyzed and their characteristic elements were identified using an EDX detection spectrometer. As shown in Fig. S2, the elements of Bi and V have almost the same percentage of atoms (%), indicating the formation of BiVO4. XRD also measured the crystalline phases of BiVO4 and BiVO4/TiO2 NTs, and the results are shown in Fig. 3. For all samples, the prominent peaks for BiVO4 are likely derived from the monoclinic phase of BiVO4 (PDF 14-0688). The typical peaks at 25.3° and 27.4° are assigned to the (101) and (110) planes of anatase and rutile phases, respectively. In Fig. 3a, the annealed composite has anatase phase and a large amount of rutile phase from the integrated intensity of the peaks associated with the (101) and (110) planes. However, for the BiVO4/TiO2(N2) NTs sample, it contains mostly anatase (Fig. 3b). These results are in accord with the reports by Jin et al. [25] and Mahajan et al. [26], who studied the effects of the atmosphere on the crystalline phase of TiO2 nanotube arrays in the annealing process. Also, the peaks at 26.4° and 37.6° for both samples are ascribed to the FTO substrate. To further study the surface composition and chemical state of TiO2(N2), XPS analysis was also conducted, and the results are illustrated in Fig. 4. The full survey indicates the presence of Sn, O, Ti, and N (Fig. 4a). Figure 4b–d shows the high-resolution XPS spectra of the elements, respectively. For the O 1s (Fig. 4b), the peak at 531.0 eV corresponds to the lattice oxygen, which is related to the Ti–O or Sn–O chemical bonding in the SnO2 or TiO2. Two distinct peaks located at 464.5 and 458.7 eV in Fig. 4c are assigned to the binding energy of Ti 2p1/2 and Ti 2p3/2, respectively, indicating the presence of Ti3+. The peak at 400.1 eV could ascribe to γ-N state, which is molecularly chemisorbed N2 [27].
The optical absorption spectra of the TiO2 NTs, TiO2(N2) NTs, BiVO4/TiO2 NTs, and the BiVO4/TiO2(N2) NTs are shown in Fig. 5. The TiO2 NTs show an absorption edge at ~360 nm, whereas, the TiO2(N2) NTs with an absorption tails extend into the visible wavelength regions. The long absorption tail indicates the presence of additional energy states within the band gap of TiO2. The energy may have resulted from the presence of oxygen vacancies or non-stoichiometric TiO2 due to annealing in a non-oxidizing atmosphere. On the other hand, the pure BiVO4 film displayed absorption within the visible region of the spectrum with the edge at ~516 nm, which corresponded to the band gap energy of 2.4 eV and further demonstrated the formation of monoclinic phase BiVO4 [28]. After the deposition of BiVO4, both the BiVO4/TiO2 NTs and the BiVO4/TiO2(N2) NTs had very similar band gap absorption compared to BiVO4, although they had enhanced intensities in the visible region. The enhanced absorption intensity was attributed to the thicker BiVO4 film in the heterojunction as observed in the SEM images.
Figure 6 presents the LSV characteristics of the TiO2 NTs, TiO2(N2) NTs, BiVO4,BiVO4/TiO2NTs, and the BiVO4/TiO2(N2)NTs, respectively. The TiO2 NTs sample exhibited a pretty low photocurrent under visible irradiation due to its large band gap, whereas the TiO2(N2) NTs sample had a slight photocurrent. The photocurrent for pure BiVO4 increased steadily with the increasing potential of the working electrode, and a photocurrent density of 1.36 mA cm−2 (1.0 V vs. Ag/AgCl) was obtained. Compared to that of pure BiVO4, a significant enhancement in photocurrent, ca. 2.06 mA cm−2 (1.0 V vs. Ag/AgCl), by the BiVO4/TiO2 NTs was observed. The photocurrent was further enhanced by approximately 30 % when using the BiVO4/TiO2(N2) NTs, which obtained the photocurrent of 2.73 mA cm−2 (1.0 V vs. Ag/AgCl). The BiVO4/TiO2(N2) with the cyclic voltammetry test also shows a stable photocurrent in the measuring range (Fig. S4).
Incident photon-to-current efficiency was measured in order to ascertain the light conversion efficiency of heterojunction of the BiVO4/TiO2(N2) NTs and was compared to the BiVO4/TiO2 NTs, BiVO4, and TiO2 in Fig. 6b. Due to a large band gap, both the TiO2 NTs and TiO2(N2) NTs had low efficiencies below 400 nm, although the TiO2(N2) NTs exhibited better performances. The IPCE of BiVO4 was comparatively at ~20 % at 410 nm, whereas heterojunction BiVO4/TiO2 NTs had a higher IPCE at nearly 28 % at 410 nm. Comparably, the IPCE of BiVO4/TiO2(N2) NTs further increased to 44 % at 410 nm, which was more than 100 % higher than the IPCE of bare BiVO4. This again suggests that the rectifying electron transfer from BiVO4 to TiO2 likely inhibits the fast recombination and increases the solar energy conversion efficiency of the junction. The IPCE was nearly zero at 550 nm, which is consistent with the optical absorption of the samples.
The PEC properties of the BiVO4/TiO2(N2) NTs were investigated by treating the organic dye (MB) under visible light illumination. It can be seen that almost no MB or little MB can be directly degraded by only applying electrocatalytic or photolytic reaction, and the TiO2 NTs only resulted in a removal ratio of only 14.1 % within 80 min, whereas the TiO2(N2) NTs had a higher efficiency of 27.2 % under the same conditions. The limited improvement in degradation of MB by TiO2 NTs was due to a large band gap that limited the use of visible light. Compared to the TiO2 NTs, the BiVO4 electrode degraded 52.4 % of the MB within the same time because of good absorption in the visible region. For the BiVO4/TiO2 NTs, the removal rate increased to 76.7 % due to fast electron transfers between the BiVO4 and TiO2 NTs. However, it is easily observed from Fig. 7a that the BiVO4/TiO2(N2) NTs obtained the removal rate of 91.8 % under the same conditions. The recycle performance of the BiVO4/TiO2(N2) NTs for PEC degradation of MB was investigated in five PEC cycles, and the results are shown in Fig. 7b. These results further suggested that the BiVO4/TiO2 NTs were stable for PEC applications, such as treating organic wastewater [29–31]. During all the process in PEC, we use 1 cm2 photoanode under visible light illumination to react.
As previously discussed, the BiVO4/TiO2(N2) NTs exhibited a significant enhancement in photoactivity as verified by higher photocurrent as well as a higher PEC efficiency in the degradation of dyes. Apparently, the TiO2(N2) NTs played an important role in the promotion of the charge transfers in the electrode. We concluded that the carrier concentration in the TiO2 NTs could be increased after annealing in a nitrogen atmosphere. To make sure the impacts of the TiO2(N2) NTs, impedance measurements were carried out at a frequency of 1 kHz on both the TiO2(N2) NTs and TiO2 NTs electrodes in 0.2 M Na2SO4 electrolytes in the dark. The results are demonstrated by the Mott–Schottky plots in Fig. 8a. From the linear portion of the Mott–Schottky plots, charge carrier densities are calculated using the relation
where N D is the charge carrier density, e is the elementary electron charge (e = 1.6 × 10−19 C), ε is the dielectric constant (ε = 48), ε 0 is the permittivity in vacuum (ε 0 = 8.85 × 10−12 F m−1), and m is the slope of the 1/C2 versus potential plot. A charge carrier density of 2.9 × 1018 cm−3 was determined for the TiO2 NTs, but was 2.1 × 1019 cm−3 for the TiO2(N2) NTs. These results indicated that the charge carrier concentration of the TiO2 NTs was indeed increased after calcination in the non-oxidizing atmospheres. The higher defect density of the nitrogen-annealed sample also involved a higher electrical conductivity [32] and rapid charge transfer.
To further confirm enhanced charge transfers between BiVO4 and the TiO2(N2) NTs in the heterojunction material, the transit time (τ d) of the majority carriers in the BiVO4/TiO2 NTs electrode and the BiVO4/TiO2(N2) NTs electrode was measured by IMPS, respectively. The transit time τ d was the average time that the photogenerated charges took to transfer to the back contact, and were estimated from the equation τ d = (2π·f min (IMPS))−1, where f min is the frequency at the minimal value in the IMPS plot. The transit time reflects the recombination probability of the photogenerated electrons and holes in the photoelectrode [33]. Figure 8b shows the IMPS plots of the BiVO4/TiO2 NTs electrode and the BiVO4/TiO2(N2) NTs electrode, respectively. According to the previous equation, the transit time τ d for the BiVO4/TiO2 NTs was 11.9, and 3.82 ms for BiVO4/TiO2(N2) NTs electrode, which indicated that the transport speed of the majority of photogenerated charges in the BiVO4/TiO2(N2) NTs electrode was three times faster than that of the BiVO4/TiO2 electrode. In other words, the BiVO4/TiO2(N2) NTs heterojunction could facilitate the majority of the photogenerated charges transported to the counter electrode and likewise, the transport of photogenerated electrons to the electrolyte is enhanced.
The transportation of electrons between the two materials was also certified by PL measurement as shown in Fig. 8c. We observed strong emission from bare TiO2 NTs and BiVO4, whereas the BiVO4/TiO2 heterojunction resulted in a near 90 % reduction in the emission intensity. The obvious quenching of luminescence of BiVO4 is characteristic of charge transfer between the BiVO4 and TiO2 NTs, implying a strong indication of the efficient reduction in recombination of charge carriers in the 1D heterojunction material. In consequence, the separation efficiency of photogenerated electron–hole pairs in BiVO4/TiO2(N2) NTs heterojunction could be improved.
Based on the experiments, We concluded that the improved performance of the BiVO4/TiO2(N2) NTs was primarily due to enhanced optical absorption and specific TiO2(N2) NTs. The nanotube structure provides larger surface area than the planar structure so that more BiVO4 photocatalyst was loaded for absorbing more visible light. On the other hand, the presence of oxygen vacancies or non-stoichiometric TiO2 in the TiO2(N2) NTs significantly enhanced the carrier density which favors the separation of photo-introduced electron–hole pairs verified by IMPS test. Thus, the higher photocurrent was obtained. The whole PEC system is shown in Fig. 9. Upon excitation by visible light, electrons were photoexcited from the valence band of BiVO4 to its conduction band. Then electron differences in the positions of the conduction bands which drove to photoelectrons generated in BiVO4 to the tubular TiO2(N2) NTs, where electrons were rapidly separated and directed to the Pt counter electrode via the external circuit. Consequently, the photogenerated electrons were scavenged by hydrogen ions on the Pt foil, and formed hydrogen gas, while the photogenerated holes oxidized water or organics on the surface of the BiVO4. Overall, the BiVO4/TiO2(N2) NTs heterojunction offered remarkable photoconversion efficiency.
4 Conclusions
A visible light response BiVO4/TiO2(N2) NTs photoelectrode was fabricated for photoelectrochemical (PEC) organic degradation. Mott–Schottky plots and IMPS demonstrated the increased carrier concentration in the TiO2(N2) NTs, which enhanced electron transfers between BiVO4 and TiO2. A photoelectrochemical measurement confirmed that the photocurrent was increased approximately 100 % using the heterojunction when compared to bare BiVO4 under 100 mW cm−2 visible light illumination. Due to its excellent photoactivity and stability, the BiVO4/TiO2(N2) NTs show a promising future in PEC applications.
References
M.G. Walter, E.L. Warren, J.R. McKone, S.W. Boettcher, Q.X. Mi, E.A. Santori, N.S. Lewis, Solar water splitting cells. Chem. Rev. 110(11), 6446–6473 (2010). doi:10.1021/cr1002326
J. Bai, J.H. Li, Y.B. Liu, B.X. Zhou, W.M. Cai, A new glass substrate photoelectrocatalytic electrode for efficient visible-light hydrogen production: CdS sensitized TiO2 nanotube arrays. Appl. Catal. B 95(3–4), 408–413 (2010). doi:10.1016/j.apcatb.2010.01.020
Z. Liu, X. Zhang, S. Nishimoto, M. Jin, D.A. Tryk, T. Murakami, A. Fujishima, Highly ordered TiO2 nanotube arrays with controllable length for photoelectrocatalytic degradation of phenol. J. Phys. Chem. C 112(1), 253–259 (2008). doi:10.1021/jp0772732
J. Bai, Y.B. Liu, J.H. Li, B.X. Zhou, Q. Zheng, W.M. Cal, A novel thin-layer photoelectrocatalytic (PEC) reactor with double-faced titania nanotube arrays electrode for effective degradation of tetracycline. Appl. Catal. B 98(3–4), 154–160 (2010). doi:10.1016/j.apcatb.2010.05.024
J.W. Tang, Z.G. Zou, J.H. Ye, Efficient photocatalytic decomposition of organic contaminants over CaBi2O4 under visible-light irradiation. Angew. Chem. Int. Ed. 43(34), 4463–4466 (2004). doi:10.1002/anie.200353594
Z.G. Zou, H. Arakawa, Direct water splitting into H2 and O2 under visible light irradiation with a new series of mixed oxide semiconductor photocatalysts. J. Photochem. Photobiol. A 158(2–3), 145–162 (2003). doi:10.1016/S1010-6030(03)00029-7
A. Kudo, Photocatalyst materials for water splitting. Catal. Surv. Asia 7(1), 31–38 (2003). doi:10.1023/A:1023480507710
Y. Park, K.J. McDonald, K.S. Choi, Progress in bismuth vanadate photoanodes for use in solar water oxidation. Chem. Soc. Rev. 42(6), 2321–2337 (2013). doi:10.1039/C2CS35260E
Y.B. Kuang, Q.X. Jia, H. Nishiyama, T. Yamada, A. Kudo, K. Domen, A front-illuminated nanostructured transparent BiVO4 photoanode for >2% efficient water splitting. Adv. Energy Mater. 6(2), 1501645 (2016). doi:10.1002/aenm.201501645
S.M. Thalluri, S. Hernandez, S. Bensaid, G. Saracco, N. Russo, Green-synthesized W- and Mo-doped BiVO4 oriented along the 040 facet with enhanced activity for the sun-driven water oxidation. Appl. Catal. B 180, 630–636 (2016). doi:10.1016/j.apcatb.2015.07.029
T.W. Kim, K.S. Choi, Nanoporous BiVO4 photoanodes with dual-layer oxygen evolution catalysts for solar water splitting. Science 343(6174), 990–994 (2014). doi:10.1126/science.1246913
L. Zhang, D.R. Chen, X.L. Jiao, Monoclinic structured BiVO4 nanosheets: hydrothermal preparation, formation mechanism, and coloristic and photocatalytic properties. J. Phys. Chem. B 110(6), 2668–2673 (2006). doi:10.1021/jp056367d
Y. Hu, D.Z. Li, Y. Zheng, W. Chen, Y.H. He, Y. Shao, X.Z. Fu, G.C. Xiao, BiVO4/TiO2 nanocrystalline heterostructure: a wide spectrum responsive photocatalyst towards the highly efficient decomposition of gaseous benzene. Appl. Catal. B 104(1–2), 30–36 (2011). doi:10.1016/j.apcatb.2011.02.031
J. Bai, B.X. Zhou, Titanium dioxide nanomaterials for sensor applications. Chem. Rev. 114(19), 10131–10176 (2014). doi:10.1021/cr400625j
X.Z. Lan, J. Bai, S. Masala, S.M. Thon, Y. Ren et al., Self-Assembled, nanowire network electrodes for depleted bulk heterojunction solar cells. Adv. Mater. 25(12), 1769–1773 (2013). doi:10.1002/adma.201203759
B. Gao, Y.J. Kim, A.K. Chakraborty, W.I. Lee, Efficient decomposition of organic compounds with FeTiO3/TiO2 heterojunction under visible light irradiation. Appl. Catal. B 83(3–4), 202–207 (2008). doi:10.1016/j.apcatb.2008.02.017
K. Sridharan, E. Jang, T.J. Park, Novel visible light active graphitic C3N4–TiO2 composite photocatalyst: synergistic synthesis, growth and photocatalytic treatment of hazardous pollutants. Appl. Catal. B 142, 718–728 (2013). doi:10.1016/j.apcatb.2013.05.077
Y. Liu, H. Zhou, J. Li, H. Chen, D. Li, B. Zhou, W. Cai, Enhanced photoelectrochemical properties of Cu2O-loaded short TiO2 nanotube array electrode prepared by sonoelectrochemical deposition. Nano-Micro Lett. 2(4), 277–284 (2010). doi:10.3786/nml.v2i4.p277-284
M.Z. Xie, X.D. Fu, L.Q. Jing, P. Luan, Y.J. Feng, H.G. Fu, Long-lived, visible-light-excited charge carriers of TiO2/BiVO4 nanocomposites and their unexpected photoactivity for water splitting. Adv. Energy Mater. 4(5), 1300995 (2014). doi:10.1002/aenm.201300995
H.F. Li, H.T. Yu, X. Quan, S. Chen, H.M. Zhao, Improved photocatalytic performance of heterojunction by controlling the contact facet: high electron transfer capacity between TiO2 and the 110 facet of BiVO4 caused by suitable energy band alignment. Adv. Funct. Mater. 25(20), 3074–3080 (2015). doi:10.1002/adfm.201500521
J. Bai, R. Wang, Y.P. Li, Y.Y. Tang, Q.Y. Zeng et al., A solar light driven dual photoelectrode photocatalytic fuel cell (PFC) for simultaneous wastewater treatment and electricity generation. J. Hazard. Mater. 311, 51–62 (2016). doi:10.1016/j.jhazmat.2016.02.052
L.E. Greene, M. Law, J. Goldberger, F. Kim, J.C. Johnson, Y.F. Zhang, R.J. Saykally, P.D. Yang, Low-temperature wafer-scale production of ZnO nanowire arrays. Angew. Chem. Int. Ed. 42(26), 3031–3034 (2003). doi:10.1002/anie.200351461
J.H. Lee, I.C. Leu, M.C. Hsu, Y.W. Chung, M.H. Hon, Fabrication of aligned TiO2 nanostructured arrays using a one-step templating solution approach. J. Phys. Chem. B 109(27), 13056–13059 (2005). doi:10.1021/jp052203l
Q.X. Jia, K. Iwashina, A. Kudo, Facile fabrication of an efficient BiVO4 thin film electrode for water splitting under visible light irradiation. Proc. Natl. Acad. Sci. 109(29), 11564–11569 (2012). doi:10.1073/pnas.1204623109
V.K. Mahajan, M. Misra, K.S. Raja, S.K. Mohapatra, Self-organized TiO2 nanotubular arrays for photoelectrochemical hydrogen generation: effect of crystallization and defect structures. J. Phys. D 41(12), 125307 (2008). doi:10.1088/0022-3727/41/12/125307
C. Jin, W.G. Zhang, S.W. Yao, H.Z. Wang, Effect of heat-treatment process on the structure and photoelectric performance of TiO2 nanotube arrays. J. Inorg. Mater. 27(1), 54–58 (2012). doi:10.3724/SP.J.1077.2012.00054
R.P. Vitiello, J.M. Macak, A. Ghicov, H. Tsuchiya, L.F.P. Dick, N-doping of anodic TiO2 nanotubes using heat treatment in ammonia. Electrochem. Commun. 8(4), 544–548 (2006). doi:10.1016/j.elecom.2006.01.023
A. Iwase, A. Kudo, Photoelectrochemical water splitting using visible-light-responsive BiVO4 fine particles prepared in an aqueous acetic acid solution. J. Mater. Chem. 20(35), 7536–7542 (2010). doi:10.1039/c0jm00961j
C. Liu, Y. Ding, W. Wu, Y. Teng, A simple and effective strategy to fast remove chromium (VI) and organic pollutant in photoelectrocatalytic process at low voltage. Chem. Eng. J. 306, 22–30 (2016). doi:10.1016/j.cej.2016.07.043
X. Zhao, J.J. Zhang, M. Qiao, H.J. Liu, J.H. Qu, Enhanced photoelectrocatalytic decomposition of copper cyanide complexes and simultaneous recovery of copper with a Bi2MoO6 electrode under visible light by EDTA/K4P2O7. Environ. Sci. Technol. 49(7), 4567–4574 (2015). doi:10.1021/es5062374
L. Liu, R. Li, Y. Liu, J. Zhang, Simultaneous degradation of loxacin and recovery of Cu(II) by photoelectrocatalysis with highly ordered TiO2 nanotubes. J. Hazard. Mater. 308, 264–275 (2016). doi:10.1016/j.jhazmat.2016.01.046
A.G. Munoz, Semiconducting properties of self-organized TiO2 nanotubes. Electrochim. Acta 52(12), 4167–4176 (2007). doi:10.1016/j.electacta.2006.11.035
J. Kruger, R. Plass, M. Gratzel, P.J. Cameron, L.M. Peter, Charge transport and back reaction in solid-state dye-sensitized solar cells: a study using intensity-modulated photovoltage and photocurrent spectroscopy. J. Phys. Chem. B 107(31), 7536–7539 (2003). doi:10.1021/jp0348777
Acknowledgments
The authors would like to acknowledge the National Nature Science Foundation of China (21507085, 21576162) and Shanghai Sailing Program of China (14YF1401500) for financial support.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Wang, R., Bai, J., Li, Y. et al. BiVO4/TiO2(N2) Nanotubes Heterojunction Photoanode for Highly Efficient Photoelectrocatalytic Applications. Nano-Micro Lett. 9, 14 (2017). https://doi.org/10.1007/s40820-016-0115-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40820-016-0115-3