Abstract
Manganese is used as a key alloying element in various advanced steel products to improve their mechanical properties. The authors have proposed an innovative process to recycle Mn from steelmaking slag. In this process, steelmaking slag is first sulfurized to separate P and Mn, and then the matte is oxidized to increase the Mn/Fe ratio. The equilibrium distribution ratio of Mn, Fe, and Ca between slag and matte has been already clarified. To design an industrial process, a kinetic model to simulate the various reactions that occur simultaneously between the matte and slag was developed by applying the coupled reaction model. In this kinetic model, the mass transfer was calculated by the double film theory by assuming the equilibrium at the interface. The calculation results agreed well with the concentration changes of each element in the matte and slag obtained by experiments. The optimum conditions for the treatment were discussed using this model, and multi-step sulfurization and oxidation processes were proposed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Manganese is used as a key alloying element in various advanced steel products to improve their mechanical properties. The annual global production of ferromanganese alloy (Fe–Mn alloy) has increased by a factor of 1.5 from 2006 to 2012, although the production of crude steel has increased only by a factor of 1.25 [1]. Thus, the increasing demand to produce advanced steels has led to an increase in Mn consumption. In Japan, the domestic production of ferroalloy has been shut down because of the high cost of electricity with the exception of high-C Fe–Mn, and the demand for high-purity Fe–Mn as an alternative to metallic Mn, which is imported from China, is increasing [1]. Because we do not have a domestic natural resource, about 70 % of Mn ore is imported from South Africa and about 20 % from Australia [2]. However, the amount imported to China is increasing exponentially, and the establishment of a stable resource is necessary. Thus, Mn has been designated as one of the national stockpile elements in Japan, and sufficient alloy for 60 days of national consumption is stored in warehouses [3]. However, Nakajima et al. [4] have studied the material flow of Mn in Japan and shown that the amount of Mn consumed as an alloying element in the steelmaking process is almost equal to that disposed in steelmaking slag [4]. Because a great amount of steelmaking slag is discarded every year, we are squandering a very useful source of Mn.
To use steelmaking slag as a valuable source of Mn, it is necessary to separate P from Mn because steelmaking slag contains about 2–5 mass % of P2O5. The authors have proposed an innovative process to recycle Mn from steelmaking slag via the sulfurization of slag because phosphorus sulfide is unstable at high temperature [5, 6]. The process begins with the sulfurization of steelmaking slag to form a Fe–Mn sulfide (matte) and to separate P. The obtained matte is then oxidized to form a Mn-rich oxide without P. High-purity Fe–Mn alloy can therefore be produced via reduction of the Mn-rich oxide phase. Fundamental experiments were performed to clarify the influences of the slag basicity and temperature on the distribution ratios of P, Mn, Fe, and Ca between the matte and slag [7–10]. The results showed that the P content in the matte was close to zero. In addition, the distribution ratio of Fe and Mn between the matte and slag increased with the slag basicity, but the distribution ratio of Ca was small for all cases. With the measured equilibrium distribution ratio and the mass balance, the authors demonstrated that most of the Mn in steelmaking slag was distributed to the matte during sulfurization, but the Mn/Fe ratio in the matte was low. However, in the oxidation treatment of the matte, which was formed by the sulfurization of slag, the Mn/Fe ratio in the oxide increased, although the distribution ratio of Mn was low [11]. Therefore, a multi-step oxidation treatment was proposed, in which the matte was oxidized several times while changing the oxide flux [12].
To design an industrial process based on these results, a kinetic model was developed to simulate the reactions between the matte and slag [13]. This paper explains the details of the kinetic model based on a coupled reaction between the matte and slag. Moreover, this model was used to determine the optimum conditions for the sulfurization of steelmaking slag to separate P and the oxidation of matte to increase the Mn/Fe ratio.
Kinetic Model for the Reactions Between Matte and Slag
During the steelmaking process, many reactions occur simultaneously. To analyze this situation, the coupled reaction model [14] is a useful method that makes it possible to calculate the reaction kinetics that occurs between the molten slag and metal phases. In this model, reactions are described using the double film theory, which assumes equilibrium conditions at the slag-metal interface. This model has been widely applied to many processes in steelmaking, i.e., hot metal pretreatment [15], BOF [16], and secondary refining [17, 18]. Many reactions occur simultaneously in the sulfurization of slag or the oxidation of matte, as shown in Fig. 1. Therefore, the basic concept of the coupled reaction model can be applied to the analysis of the reaction kinetics between the matte and slag. In the case of reaction between the slag and metal, the oxidation reaction of element “M” is given in Eq. 1, and the molar flux density is expressed as a function of the oxygen activity at the interface.
Based on this concept, the reaction of the oxide and sulfide of the element “M” is given in Eq. 2 for the case of the slag and matte reaction:
The molar flux density of the cation M (J M; mol/m2/s) can be represented by Eq. 3.
where k S and k M are the mass transfer coefficients in the slag and matte (m/s), respectively, and ρ S and ρ M are the densities of the slag and matte phases (kg/m3), respectively. NMO and NMS are the molar weights of MO and MS, respectively. By assuming equilibrium at the interface, the relationship between (%MO)* and (%MS)* can be represented by Eq. 4.
In this equation, K1 is the equilibrium constant, and \( {\text{P}}_{{{\text{O}}_{ 2} }} {*}/{\text{P}}_{{{\text{S}}_{ 2} }} * \) is the ratio of the partial pressures of oxygen and sulfur at the interface. These equations are written for all cations in the system. Because the interfacial content can be deleted by the combination of Eqs. 3 and 4, J M can be described as a function of the ratio of the partial pressures of oxygen and sulfur at the interface \( {\text{P}}_{{{\text{O}}_{ 2} }}^*/{\text{P}}_{{{\text{S}}_{ 2} }}^* \). When the equations for J M of every cation are inserted into Eq. 5, which indicates the condition for electric neutrality, the value of \( {\text{P}}_{{{\text{S}}_{ 2} }} /{\text{P}}_{{{\text{O}}_{ 2} }} \) at the interface can be evaluated. Then, the concentration change for all cations in the slag and matte phases can be calculated by using the evaluated value of \( {\text{P}}_{{{\text{O}}_{ 2} }}^*/{\text{P}}_{{{\text{S}}_{ 2} }}^* \). The difference between this calculation and the calculation for slag and metal system is that the molar flux density is calculated as a function of \( {\text{P}}_{{{\text{O}}_{ 2} }}^*/{\text{P}}_{{{\text{S}}_{ 2} }}^* \) instead of the oxygen activity at the interface.
For the calculation, the precise estimation of the activity coefficients of oxide and sulfide is imperative. The authors have already confirmed [9, 10] that the activity coefficient of MO (γ MO) in slag can be calculated using a regular solution model [19]. The equilibrium relation between matte and slag, which was measured in a previous paper [10], can be used to evaluate the activity and the activity coefficient of sulfide. However, a reliable calculation model of the activity coefficient of sulfide in matte has not been proposed. Therefore, here, the activity coefficients of MnS, FeS, and CaS (γ MnS, γ FeS, and γ CaS) were formulated as a function of \( {\text{P}}_{{{\text{O}}_{ 2} }} /{\text{P}}_{{{\text{S}}_{ 2} }} \) according to the experimental results [13], and the following equations were obtained.
Theoretically, the activity coefficient is determined by the composition and temperature, but a reliable relation was not obtained by a regression analysis of the experimental results. In this experiment, the matte was not pure sulfide but oxi-sulfide, and the influence of the oxygen content of the matte on the activity coefficient was considerable. Therefore, in this study, the activity coefficient was formulated as a function of \( {\text{P}}_{{{\text{O}}_{ 2} }} /{\text{P}}_{{{\text{S}}_{ 2} }} \), which affects the oxygen content in the matte.
In addition, the mass transfer coefficients of slag and matte (k S and k M) have to be determined for this calculation. In the case of metal and slag, the mass transfer coefficient of metal is generally 3–10 times larger than that of the slag [14, 20]. The viscosity of matte is about 10–100 times larger than that of slag [21, 22], and the diffusion coefficient in matte is about 10 times larger than that in slag. However, the viscosity of molten steel is about 100 times larger than that of slag [21], and the diffusion coefficient in molten steel is about 100 times larger than that in slag [21, 23]. Therefore, the ratio of the mass transfer coefficient between slag and matte is assumed to be smaller than the ratio between molten steel and slag. Because the ratio of the mass transfer coefficient between molten steel and slag is in the range of 3–10, it is reasonable to use the same values for k S and k M. The value of k S (and k M) is determined to fit the calculation curve with the experimental result. The difference in the values for each cation was not considered.
To apply this model to the laboratory-scale experiment, the dissolution rate of MgO from the crucible to the slag has to be considered because the interfacial area between the crucible and the slag is relatively large. Generally, the mass transfer of MgO in slag is assumed to be the rate-controlling step for the dissolution of MgO from a refractory to slag [23]. In this case, Eq. 9 can be written as follows:
Given this equation, the dissolution of MgO in the slag phase can be described by the following equation.
In Eq. 9, A is the interfacial area between the slag and the refractory material (m3), kMgO is the mass transfer coefficient of MgO in slag (m/s), and V is the volume of slag (m3). In addition, (% MgO)Sat. and (% MgO)Init. indicate the saturated and initial MgO contents, respectively. In this study, (% MgO)Sat. was determined as a function of the sum of the FeO and MnO contents in the slag, which were experimentally obtained in our previous research [10]. The value of Ak/V in Eq. 10 was determined such that the calculation results agreed well with the experimentally observed changes in the MgO content, as shown in Fig. 2.
Experimental Procedure
Table 1 lists the compositions of the matte and the slag before the experiment. Experiment C simulated the sulfurization of steelmaking slag, and experiments A and B simulated the oxidation of the matte. The slags for A and B were melted in a SiO2 crucible for 1 h for preparing SiO2-saturated liquid slag, because, according to the equilibrium experiment, the acid slag increased the Mn/Fe ratio [10]. After pre-melting, matte and slag were quenched in water and crushed. Steelmaking slag for C was prepared by a mixture of reagent-grade MnO, MgO, Ca3(PO4)2, SiO2, CaO, Fe, and Fe2O3 powder. CaO was prepared by the calcination of CaCO3 at 1273 K for 2 h, and FeO was prepared by heating a mixture of Fe2O3 and Fe powders (mole ratio: 1:1) in an iron crucible under pure argon atmosphere at 1673 K for 1 h. Mattes for A and B were produced by mixing CaO and FeO with a reagent of FeS and MnS in an appropriate ratio. For C, the CaS–FeS matte was used, where CaS was formed by the reduction of CaSO4 with carbon. To prepare the matte, first, a mixture of reagent-grade CaSO4 and carbon powder was prepared in an appropriate mole ratio (1:4). Then, half of the amount of FeS was put in an MgO crucible, and a mixture of CaSO4 and C was charged on the FeS in the crucible. Finally, after charging residues of FeS, the matte sample and the crucible were heated at 1723 K under Ar for 3 h.
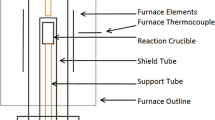
In order to investigate the kinetic reaction between matte and slag, 7.5 g of was placed in a MgO crucible (19 mm in inner diameter and 45 mm in height), and 3 g of slag was placed on the matte. The atmosphere in the furnace was maintained by blowing Ar gas. The reaction time was set to 600–2400 s. After holding for a given time, the sample was withdrawn from the furnace and quenched using He gas. The quenched samples were then ground, and the obtained matte and slag powder were collected carefully. The Fe, Mn, Mg, P, and Ca contents of the matte and slag were analyzed by inductively coupled plasma atomic emission spectroscopy (ICP-AES). The concentrations of SiO2 were analyzed by the alkali-fusion method and a gravimetric method.
Results
Figures 3, 4, and 5 show experimental results for the slag and matte obtained from experiments A, B, and C, respectively. These figures also show the calculation results given by the kinetic model. For these calculations, the value of k S (or k M) was determined such that the calculation line agreed with the experimental result. The actual calculated value of k M was 0.0015 m/s, which is close to that used for the slag/metal reaction in a laboratory-scale experiment [20]. The overall mass transfer coefficient (k ov) is described by the following equations, where L is the equilibrium distribution ratio and X M and X S are the mole fractions of X in the matte and slag, respectively.
In this case, the value of L was about 250 because E M in Eq. 4 for each reaction was about 0.002 and \( ({\text{P}}_{{{\text{S}}_{ 2} }} /{\text{P}}_{{{\text{O}}_{ 2} }} )^{ 1/ 2} \) was about 0.2. Therefore, under the assumption that k S = k M, the reaction rate is strongly controlled by the mass transfer in the matte.
As shown in Figs. 3a and 4a, the FeO content in the slag decreased, whereas the MnO and CaO contents increased with the reaction time. In contrast, the Mn and Ca contents in the matte decreased, and the Fe content increased, as shown in Figs. 3b and 4b. The change in the SiO2 content in the slag was not remarkable because Si oxide is much more stable than Si sulfide. This implies that the oxidation of Mn in the matte was caused by FeO in the slag. As mentioned above, the MgO content in the slag increased because of the dissolution of the MgO crucible. On the other hand, Fig. 5 shows the reaction between the Fe–Ca matte and the steelmaking slag. Our previous studies [7–10] confirmed that Si, Mg, and P were hardly distributed to the matte. According to the chemical analysis, the concentrations of Mg and Si in the matte were below 0.5 mass %, and the P content was below 0.1 mass %. This implies that these elements were not involved in the reaction between the matte and the slag. Therefore, the concentrations of SiO2 and P2O5 in the slag were almost constant, as shown in Fig. 5a; the increment in MgO was caused by the dissolution of the MgO crucible. In addition, the concentrations of MnO and FeO decreased and that of CaO increased with time. As shown in Fig. 5b, the Mn in the matte increased and the CaS decreased with time. This indicates that the MnO in the slag can be sulfurized by CaS–FeS matte.
Because the mass balance of the experimental result was not accurate, it was difficult to fit the calculation curve to all data of the experiment. The reason for the disagreement between the mass balances is unclear, but it may be due to the emulsion of matte particles in the slag phase (and vice versa) or the non-uniform condition of the liquid phases. However, the general trend of the composition change can be calculated using this model. By using the developed model, the optimum conditions for the sulfurization of slag and the oxidation of matte can be determined.
Discussion
Sulfurization of Slag
In the proposed process to recover Mn from steelmaking slag, the first step is the sulfurization of slag. In this simulation, 1000 kg of steelmaking slag, which had the composition shown in Table 1, was sulfurized by the addition of matte. The initial compositions and added mass are shown in Table 2. The treatment temperature and time were 1723 K and 3600 s, respectively. The diameter of the furnace was assumed to be 1 m, and the interfacial area was calculated geometrically. The mass transfer coefficients of both the slag and the matte were assumed to be 0.002 m/s. This value was calculated using the empirical equation originally proposed to calculate the mass transfer coefficient of the metal phase under Ar gas bubbling [20]. The Mn yields calculated by Eq. 12 and the Mn content in matte after the treatment ({Mn}) were evaluated.
where \( W.{\text{Mn}}_{\text{Slag}}^{\text{Initial}} \) and W.MnMatte denote the initial mass of Mn in slag and the mass of Mn in matte after the treatment, respectively. Figure 6 shows the influence of the ratio of the initial masses of slag and matte (R S/M) on the Mn yield and {Mn} after the treatment by each matte with a different initial composition. When the weight ratio of the slag and matte was constant, the Mn yields increased with the CaS content in the initial matte, but the increments of {Mn} were insignificant. However, {Mn} increased with an increase in R S/M even though the Mn yields were in inverse proportion to R S/M. The Mn yield is related to the distribution ratio between the matte and the slag and can be improved by increasing \( \left( {P^{*}_{{{\text{S}}2}} /P^{*}_{{{\text{O}}2}} } \right) \). Figure 7 represents the simulated ratio of \( {\text{P}}_{{{\text{O}}_{ 2} }} \;{\text{and}}\;{\text{ P}}_{{{\text{S}}_{ 2} }} \) at the interface as a function of time by changing R S/M when the ratio of CaS and FeS was constant at 0.25. The ratio of \( \left( {P^{*}_{{{\text{S}}2}} /P^{*}_{{{\text{O}}2}} } \right)^{1/2} \) increased with time and a decrease in R S/M. This implies that the Mn yield can increase with the mass of matte because of an increase in \( \left( {P^{*}_{{{\text{S}}2}} /P^{*}_{{{\text{O}}2}} } \right) \). For the effective recovery of Mn from steelmaking slag, the ratio of Mn to Fe in the product must be increased after sulfurization. In this purpose, the control of \( {\text{P}}_{{{\text{S}}_{ 2} }} /{\text{P}}_{{{\text{O}}_{ 2} }} \) at the interface at the appropriate value is the most important factor. In the industrial process, \( {\text{P}}_{{{\text{S}}_{ 2} }} \) and \( {\text{P}}_{{{\text{O}}_{ 2} }} \) are determined by the mass ratio of the slag and matte, the additional rate of flux, the stirring condition, etc. However, in this experimental condition, the slag/matte ratio has the greatest effect on this value.
When used matte was added to fresh steelmaking slag repeatedly, it is expected that the {Mn} content would increase, maintaining a high Mn yield. Figure 8 and Table 3 present the concept of this multi-step sulfurization process and the simulation conditions for each step, respectively. In this table, the initial matte compositions were obtained from the simulation result of the previous step. When the fresh steelmaking slag was charged on the matte N times, the Mn yield can be expressed as follows:
In the case shown in Fig. 9, the value of N is 4. As shown in Fig. 9, by carrying out the multi-step treatment, the Mn yield and {Mn} increased remarkably compared with a single-step treatment.
Oxidation of Matte
Despite the multi-step treatment of sulfurization, the Mn content of the matte was not sufficiently high to produce a Fe–Mn alloy. Therefore, it is important to form a Mn-rich oxide phase by oxidation of the matte. The authors have already proposed a multi-step oxidation treatment based on thermodynamic data [12] to increase the ratio of Mn/Fe in the oxidized slag with a high Mn yield. The kinetic model was applied to the multi-step oxidation treatment. In this process, first, CaS in the matte is preferentially oxidized to form MnS–FeS matte (Ca removal step). Then, the residual matte is oxidized several times by changing the oxidized flux to increase the Mn/Fe ratio in the slag (Mn oxidation step), as shown in Fig. 10.
Initially, the Ca removal step was calculated. Table 4 lists the compositions and masses of the oxidized flux and matte before the Ca removal step. The compositions of the initial oxidized flux were determined to form a liquid phase in the FeO–MgO–SiO2 ternary system [24, 25] by assuming that the MgO content is 15 mass %, and the mass ratio of the oxidized flux to the matte and the ratio of FeO/SiO2 changed. In this simulation, 2000 kg of matte formed by sulfurization treatment was oxidized by the addition of flux. The treatment temperature and time were 1723 K and 3600 s, respectively. The diameter of the furnace was 1 m, and the mass transfer coefficient was calculated by the same method used for the sulfurization treatment.
Figure 11 shows the influence of R S/M on {Mn} and {Ca} in the matte and the removal ratio of element “M” (%) after the treatment. The residual ratio is determined by the following equation.
where W.M InitialMatte and W.MMatte denote the mass of element M in the matte before and after oxidation, respectively. It is clear that {Ca} decreased as R S/M increased, and the removal ratio of Ca was close to 100 % when R S/M increased to 0.8 or more. However, the removal ratio of Mn increased when R S/M increased to 0.8 or more. Figure 12 shows the influence of FeO/SiO2 when R S/M was 0.8. The removal ratio of Ca increased as the FeO/SiO2 ratio increased and then decreased when the FeO/SiO2 ratio increased to 0.7 or more. The removal ratio of Mn gradually increased as the FeO/SiO2 ratio increased. Therefore, as the initial flux, a value of 0.8 for R S/M and 0.7 for FeO/SiO2 are the optimum conditions for the preferential oxidation of CaS in the matte.
Then, the optimum conditions for Mn oxidation step to produce the oxidized slag with high Mn/Fe ratio were determined. In this simulation, 2000 kg of matte formed after the Ca removal step was oxidized by the addition of 1000 kg of oxide (R S/M is 0.5). The treatment temperature, time, and diameter of the furnace were the same as those used in the Ca removal step. In the Mn oxidation step, the flux to oxidize the matte was discharged, and a fresh flux of oxide was added several times to avoid the decrease in the Mn/Fe ratio of the oxidized slag. Table 5 lists the compositions of the oxidized flux and matte for the calculation. The matte composition of A was determined by the calculation result of the Ca removal step. The matte composition of A-III and A-V indicates that the composition after the oxidation treatment was repeated 3 and 5 times, respectively. In each treatment, R S/M was 0.5, and FeO/SiO2 was 0.3. The treatment time and temperature were the same as in the other cases.
By changing the ratio of FeO/SiO2 in the oxidized slag, the mass ratio of MnO/FeO (R MnO/FeO) and Mn yields were evaluated as follows:
where W.MnOxidized slag and W.FeOxidized slag denote the mass of Mn and Fe in the slag after the oxidized treatment, respectively. As shown in Fig. 13, as the number of repetitions increased from 1 to 5, R MnO/FeO increased. Therefore, the multi-step oxidation treatment is an effective measure to produce oxidized slag with a high Mn/Fe ratio for the raw material of the Fe–Mn alloy. The Mn yield of each step was not high, and it decreased with the number of repetitions. This indicates that, in each step, only about 30 % of Mn was oxidized, but the residual Mn in the matte was oxidized in the following steps until it decreased to a sufficiently low level. In every case, as the FeO/SiO2 ratio decreased, R MnO/FeO increased linearly, but the Mn yield decreased when the ratio decreased to 0.3 or less. Therefore, the optimum ratio of FeO/SiO2 in the oxidized slag is considered to be about 0.3.
Conclusion
Because the amount of Mn consumed as an alloying element in the steelmaking process is almost equal to the amount of Mn disposed in steelmaking slag in Japan, the authors propose an innovative process to recycle Mn from steelmaking slag. In this process, first, steelmaking slag is sulfurized to separate P and Mn, and then the matte is oxidized to increase the Mn/Fe ratio. The equilibrium distribution ratio of Mn, Fe, and Ca between the slag and matte has already been clarified. To design an industrial process, a kinetic model to simulate the various reactions that occur simultaneously between the matte and slag was developed by applying the coupled reaction model, which was originally developed to calculate the reaction kinetics that occur between the molten slag and the metal phases. In this model, reactions are described using the double film theory, while assuming equilibrium conditions at the slag-metal interface, and the molar flux density is expressed as a function of the oxygen activity at the interface. This model was applied to the reaction kinetics between the molten slag and matte, and in this case the molar flux density was expressed as a function of the ratio of the partial pressures of oxygen and sulfur at the interface.
The calculation results agreed well with the concentration changes of each element in the matte and slag obtained by the experiments. With this model, the optimum conditions for the treatment were discussed, and a multi-step sulfurization and oxidation treatment were proposed.
References
Nippon Donko Co. Ltd. (2015) Nippon Denko Daijitenn, website of Nippon Donko Co. Ltd. http://www.nippondenko.co.jp/company/index.html. Accessed 20 Sep 2015
Japan Oil, Gas and Metals National Corporation (JOGMEC) (2014) Material flow of mineral resources, website of JOGMEC. http://mric.jogmec.go.jp/public/report/2015-03/14_201504_Mn.pdf. Accessed 20 Sept 2015
Japan Oil, Gas and Metals National Corporation (JOGMEC) (2014) Rare Metals Stockpiling Program, website of JOGMEC. http://www.jogmec.go.jp/english/stockpiling/stockpiling_015.html. Accessed 20 Sept 2015
Nakjima K, Yokoyama K, Nagasaka T (2008) Substance flow analysis of manganese associated with iron and steel flow in Japan. ISIJ Int 48(4):549–553
Kim S-J, Hotta T, Shibata H, Kitamura S, Yamaguchi K (2010) Fundamental research to produce ferro-manganese alloy from steelmaking slag. In: Proceedings of the 6th European Slag Conference, UNESID, Madrid, Spain, pp 183–191
Kitamura S, Shibata H, Kim S-J, Teradoko T, Maruoka N, Yamaguchi K (2011) Novel process for recycling valuable elements from steelmaking slag. In: Proceedings of the 2nd International Slag Valorization Symposium, KU Leuven, Leuven, Belgium, pp 329–340
Kim S-J, Shibata H, Maruoka N, Kitamura S (2011) Yamaguchi K (2011) Novel recycling process of Mn by sulfurization of molten slag from a by-product of steelmaking process. High Temp Mater Process 30(4,5):425–434
Kim S-J, Shibata H, Takekawa J, Kitamura S, Yamaguchi K, Kang Y-B (2012) Influence of partial pressure of sulfur and oxygen on distribution of Fe and Mn between liquid Fe-Mn oxysulfide and molten slag. Metall Mater Trans B 43B(5):1069–1077
Kim S-J, Takekawa J, Shibata H, Kitamura S, Yamaguchi K, Kang Y-B (2013) Thermodynamic assessment of MnO and FeO activities in FeO-MnO-MgO-P2O5-SiO2(-CaO) molten slag. ISIJ Int 53(8):1325–1333
Kim S-J, Takekawa J, Shibata H, Kitamura S, Yamaguchi K (2013) Influence of slag basicity and temperature on Fe and Mn distribution between liquid Fe-Mn-Ca-O-S Matte and Molten Slag. ISIJ Int 53(10):1715–1724
Kim S-J, Shibata H, Kitamura S, Yamaguchi K (2013) Innovative process of manganese recovery from steelmaking slag by sulfurization. In: Proceedings of high temperature processing symposium 2013, Swinburne University, Melbourne, Australia, pp 68–70
Kim S-J (2013) Fundamental research on the recovery of manganese from steelmaking slag. Doctoral dissertation of Tohoku University
Kim S-J, Kitamura S (2014) Extraction of Mn from steelmaking slag to produce Fe-Mn alloy. CAMP-ISIJ 27(1):149–152
Oguchi S, Robertson DGC, Deo B, Grieveson P, Jeffes JHE (1984) Simultaneous dephosphorization and desulphurization of molten pig iron. Ironmak Steelmak 11(3):202–213
Kitamura S, Kitamura T, Shibata K, Aida E, Sakomura R, Kaneko T, Nuibe T (1991) Development of analysis and control method for hot metal dephosphorization process by computer simulation. ISIJ Int 31(11):1329–1335
Knoop W, Deo B, Snoeijer A, Unen G, Boom R (1992) A dynamic slag-droplet model for the steelmaking process. In: Proceedings of the 4th international conference on molten slags and fluxes, ISIJ, Tokyo, pp 302–307
Graham KJ, Irons GA (2008) Couples kinetic phenomena in ladle metallurgy. In: Proceedings of the 3rd international conference on process development in iron and steelmaking (SCANMET III), MEFOS, Lulea, Sweden, vol 1, pp 385–396
Harada A, Maruoka N, Shibata H, Kitamura S (2013) A kinetic model to predict the compositions of metal, slag and inclusions during ladle refining: part 1. Basic concept and application. ISIJ Int 53(12):2110–2217
Banya S (1993) Mathematical expression of slag-metal reactions in steelmaking process by quadratic formalism based on the regular solution model. ISIJ Int 33(1):2–11
Kitamura S, Kitamura T, Shibata K, Mizukami Y, Mukawa S, Nakagawa J (1991) Effect of stirring energy temperature and flux composition on hot metal dephosphorization kinetics. ISIJ Int 31(11):1322–1328
Japan Institute of Metals (2004) Kinzoku data book, 4th edn. Maruzen, Tokyo, pp 55–87
Vaisburd S, Brandon DG, Kozhakhmetov S, Kenzhaliyev E (2002) Physicochemical properties of molten-slag melts taken from Vanyukov’s furnace for copper extraction. Metall Mater Trans B 33B(8):561–564
Nagata K, Sata N, Goto K (1982) Diffusivities in molten slag molten iron, steel and refractories. Tetsu-to-Haganè 68(13):1694–1705
Umakoshi M, Mori K, Kawai Y (1981) Dissolution rate of sintered MgO into molten FetO-CaO-SiO2 slags. Tetsu-to-Haganè 67(10):1726–1734
Verein Deutscher Eisenhüttenleute (1981) Slag atlas, 2nd edn. Verlag Stahleisen GmbH, Dusseldorf, Germany, p 142
Acknowledgments
The authors appreciate the financial support from the Grant-in-Aid for Japan Society for the Promotion of Science, a Grant-in-Aid for Scientific Research (B) (26289278).
Author information
Authors and Affiliations
Corresponding author
Additional information
The contributing editor for this article was B. Mishra.
Rights and permissions
About this article
Cite this article
Kim, SJ., Suzuki, J., Gao, X. et al. A Kinetic Model to Simulate the Reaction Between Slag and Matte for the Production of Ferromanganese Alloy from Steelmaking Slag. J. Sustain. Metall. 2, 141–151 (2016). https://doi.org/10.1007/s40831-016-0042-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-016-0042-z