Abstract
NdFeB permanent magnets have different life cycles, depending on the applications: from as short as 2–3 years in consumer electronics to 20–30 years in wind turbines. The size of the magnets ranges from less than 1 g in small consumer electronics to about 1 kg in electric vehicles (EVs) and hybrid and electric vehicles (HEVs), and can be as large as 1000–2000 kg in the generators of modern wind turbines. NdFeB permanent magnets contain about 31–32 wt% of rare-earth elements (REEs). Recycling of REEs contained in this type of magnets from the End-of-Life (EOL) products will play an important and complementary role in the total supply of REEs in the future. However, collection and recovery of the magnets from small consumer electronics imposes great social and technological challenges. This paper gives an overview of the sources of NdFeB permanent magnets related to their applications, followed by a summary of the various available technologies to recover the REEs from these magnets, including physical processing and separation, direct alloy production, and metallurgical extraction and recovery. At present, no commercial operation has been identified for recycling the EOL NdFeB permanent magnets and the recovery of the associated REE content. Most of the processing methods are still at various research and development stages. It is estimated that in the coming 10–15 years, the recycled REEs from EOL permanent magnets will play a significant role in the total REE supply in the magnet sector, provided that efficient technologies will be developed and implemented in practice.
Similar content being viewed by others
Introduction
Neodymium–iron–boron (Nd2Fe14B, or NdFeB for short) permanent magnets are considered as the best available magnets since their introduction on the market in 1984, due to their superior energy product (with a theoretical maximum of 512 kJ/m3), which makes them highly efficient and suitable for lightweight mobile applications [1]. Consequently, they are widely used in wind turbines, hybrid and electric vehicles (HEVs and EVs), household electrical appliances, computer hard disk drives (HDDs), and many small consumer electronic devices.
NdFeB permanent magnets have different life cycles, depending on the applications: from as short as 2–3 years in consumer electronics to 20–30 years in wind turbines. The weights of the magnets range from less than 1 g in small consumer electronics to over 1 kg in EVs and HEVs, and can be as large as 1000–2000 kg in the generators of modern wind turbines. NdFeB magnet contains about 31–32 wt% rare-earth elements (REEs), mainly Nd + Pr plus a few minor, but expensive, heavy rare-earth elements (HREEs) such as Dy, Tb, and Gd, depending on the applications. Recycling of REEs contained in this type of magnets from the End-of-Life (EOL) products will play an important and complementary role for the primary supply of REEs in the future. However, collection and recovery of the magnets from small consumer electronics imposes great social and technological challenges. At present, no mature and economically feasible technologies have been identified for recycling the EOL NdFeB permanent magnets and the recovery of the associated REEs. Most of the processing methods are still at the research and development stage. It is estimated that from 2030 on, the recycled REEs from the EOL permanent magnets will play a significant role in the total REE supply in the magnet sector [2]. Moreover, recycling of REEs is also of importance for the so-called Balance Problem, since it avoids producing excesses of La or Ce while mining of REE ores for Nd and/or Dy production [3–5].
To date, no commercial process to recycle these REEs from EOL products is operational. It is simply cheaper to buy REE master alloys or newly manufactured magnets originated from primary resources than to reprocess the complex scrap material from recycled resources [6, 7], especially for small-sized products where manual separation of the magnets is challenging. Moreover, since most household electrical and electronic devices are currently shredded, their magnetic content, mainly containing iron and neodymium, tends to end up in the ferrous metal waste stream, which is too diluted for economically feasible REE recovery.
There are different metallurgical processes to recover the REEs in the NdFeB magnet scrap at various technology-readiness levels (TRLs) [8–11], including hydrogen decrepitation [12, 13], chemical vapor transport [14, 15], liquid metal extraction [16], hydrometallurgical processing [17, 18], and pyrometallurgical slag extraction [19], among many others. However, the majority of these techniques were developed to recover the REEs from pre-consumer (manufacturing) scrap that is relatively clean and homogenous, and particularly having a high REE concentration. The recovery of REEs from complex post-consumer magnet scrap has not been well studied before. This paper gives an overview on the sources of EOL permanent magnets and characteristics of their occurrence, technical challenges and potential physical and metallurgical separations for the recovery of the magnets and the associated REEs, and finally a life cycle assessment (LCA) for recycling EOL NdFeB magnet scrap.
Applications of NdFeB Magnets and the Source for Future Recycling
NdFeB Magnet Production and Market Demand
The global annual REE production remained rather constant at about 120,000 tons (counted as oxides-REOs) during 2005–2015, except for two peak years of 2006 and 2009 (~137,000 tons) according to the USGS Minerals Yearbook for Rare Earths published annually during 2010–2016 [20]. Although very little public statistics are available for individual REE production in the past years, a thorough analysis of the global distribution of rare-earth oxide consumption by market sectors was reported for the year 2008 by the U.S. Department of the Interior and the USGS [21]. Approximately 26,000 tons of REEs (counted as REOs) are used per year in NdFeB permanent magnets, which form the largest applications among all REEs both in tonnage and in market value [22, 23], as shown in Fig. 1. However, it is very difficult to get accurate and reliable REE production figures for the use of the NdFeB magnets in the different applications, and thus many statistics are based on estimations from different sources and may differ significantly from one source to the other.
Market shares of REE magnets in terms of volume and monetary value (created based on data of BGS [22])
The great versatility of the REE magnet applications is also contributed by the fact that fine-tuning their chemical composition by adding some Dy, Tb, Gd, Nb, Co, Cu, Ga, and Al can modify some of the physical and magnetic properties to suit wide application needs [24]. Dy and Tb are used to increase the anisotropy and the coercivity of the magnet, which is of importance for magnets to be used at higher temperatures, but they also decrease the remanence and the energy product. Addition of Gd improves the temperature coefficient. Cu and Al are added to improve sintering of the magnet alloy, while Nb is added for grain refining. Ga improves the intrinsic coercivity and the hot workability of the alloy. Co is added to increase the Curie temperature, and some NdFeB magnets could contain up to 5 wt% Co. In 2008, about 26,300 tons of REEs including Nd, Pr, Dy, Gd, and Tb, counted as oxides, were used for magnet production globally. For the 26,300 tons of REOs above, about 76 % of neodymium (18,200 vs. 23,900 tons), 70 % praseodymium (6140 vs. 8740 tons), 69 % gadolinium (525 vs. 762 tons), and 100 % dysprosium (1310 tons) have been used for permanent magnet production in 2008 [21].
Figure 2 shows the predicted growths of all REEs according to the application areas [25]. As seen from the figure, the REE magnets and dysprosium will be the most demanded materials, largely due to the fast growing green technologies for clean energy and transportation (wind turbines and electric vehicles).

Adapted from Alnoso et al. [25] (reprinted with permission)
Predicted growth of REE demand (2010–2035)
REE permanent magnet contains about 31–32 wt% REEs (mainly 21–31 wt% (Nd + Pr), 0–10 wt % Dy plus small amounts of Gd and Tb) [26]. Dysprosium is essential for high-temperature applications such as in EVs/HEVs. It is expected that the NdFeB permanent magnet market will continue to grow, and Fig. 3 illustrates the recent global NdFeB permanent magnet production and forecast to 2017/2020 [24], and Table 1 lists the magnet production in year 2012 and prediction for the year 2015 [27]. As clearly illustrated in Fig. 3 and Table 1, Japan and China have been the two main REE magnet-producing countries, and in recent years, China has been by far the dominant magnet-producing country with about 80 % market share. According to the 2013 forecast in the UNEP report, the REO demand for REE magnet production will reach 36,000 tons, an equivalent of NdFeB magnet of approximately 99,500 tons [28]. However, the predicted figures for 2015 and 2016 may differ from the real demand and production significantly due to the weakened market demand in the recent years. Unfortunately, there exist no public statistics on the total NdFeB permanent magnet production and demand for the recent years to validate the former predictions. The known applications using NdFeB magnet (incomplete) are likely to come down showing a much lower figure as will be discussed in “Applications of NdFeB Magnets” section.
Global production of NdFeB permanent magnets and the demand for the REEs [23] (reprinted with permission). a Total global NdFeB magnet production and prediction: 2005–2020, b Total global REE demands for permanent magnets
Manufacturing and Applications of NdFeB Magnets
Properties and Production Methods of Rare-Earth Permanent Magnet (REPM)
Today, key drivers for the development of new permanent magnet materials are the growing demands for industrial motors and automation, consumer electronics, e-mobility, and the shift toward renewable energies, most importantly wind power [29]. As a consequence of the 2010/2011 Rare Earth Crisis, research has also been driven by the quest for permanent magnet materials without [30] or with a reduced amount of critical rare-earth metals [31]. There is a paradigm shift in how this research problem is approached: while so far the impressive advances in permanent magnet research have almost exclusively been pushed by experimental materials science research, today, the modeling of new materials systems gains more importance [32].
Making a permanent magnet is governed by following two major principles: first, choosing the right starting material that comprises good intrinsic properties, that is, saturation magnetization, Curie temperature, and magnetocrystalline anisotropy; second, translating these intrinsic properties into functional extrinsic properties, most importantly a high remanent magnetization and coercivity (hysteresis). This is done by processing the materials appropriately and adjusting respective microstructures [33]; a schematic of main processing routes can be found in Fig. 4. In the case of Nd–Fe–B, the market is dominated by sintered, the so-called nucleation-type magnets. In this case, a Nd–Fe–B starting alloy is produced by a strip caster, which is used to cast the alloy on a water-cooled, rotating wheel that rapidly quenches the alloy. The great advantage of this technique is that the resulting alloy comprises a very homogenous microstructure with Nd–Fe–B grains in the microscale; the segregation of α-Fe is largely avoided. In a second step, the material is pulverized by hydrogen decrepitation (HD) and then jet-milled down to a grain size of typically 3–5 µm [34]. The powder is then aligned in a magnetic field and pressed to make a textured green compact, which is then sintered. The overall REE content of the magnet is considerably higher as suggested by the stoichiometry of the main phase: the extra REE portion makes it possible to run a liquid-phase sintering process. The sintered body is then finally annealed to improve the magnetic properties, most importantly the coercivity. The REE-rich, intergranular low-melting eutectic phases are the key to enhance coercivity, that is, the ability of the magnet to withstand demagnetization: the idea is to magnetically decouple the grains in order to avoid the movement of transverse domains as much as possible. The magnets are mechanically worked (sawing, grinding, polishing) to get a final product. In terms of (raw materials) cost, this final production step should not be underestimated: many tons of REE-rich grinding slurry are accumulated each year, a valuable resource, in respect of which an appropriate, cost-effective, and clean recycling process is still a challenge.
Magnetization reversal, i.e., the nucleation of domains after the magnet has first been saturated and mounted and then been exposed to an opposing magnetic field in the application, happens by nucleation at “imperfect” interfaces of individual Nd–Fe–B grains (“nucleation-type magnet”)—“imperfect” in the sense of magnetic, structural, chemical, or geometrical imperfections [35]. This observation led to the development of the so-called grain boundary diffusion process, in which HREEs, most importantly Dy and Tb, are diffused into the microstructure of the magnet along the grain boundaries, magnetically hardening particularly these areas of the grains (Dy and Tb comprise higher magnetocrystalline anisotropies than Nd). Apart from the cost-wise benefits, the reduced addition of HREEs increases the remanence of highly coercive Nd–Fe–B, thus the energy density, (BH)max, due to the lower amounts of HREE 4f spins that couple antiparallel with the 3d electrons. Interestingly, this kind of advanced processing experienced its industrial breakthrough in the aftermath of the 2010/2011 global Rare Earth Crisis, when prices particularly for HREE oxides were multiplying by several orders of magnitude.
The second major class of Nd–Fe–B permanent magnets are nanocrystalline, polymer-bonded magnets. The main steps of the production route adopted are (1) rapidly quenching a Nd–Fe–B alloy by melt spinning, that is, at much higher solidification speeds than those achieved by a strip caster, to produce polycrystalline flakes with grains in the nanoscale; (2) milling these flakes to a standardized particle size; and (3) mixing the flakes with a polymer and injecting or pressing this compound into a magnet. The major advantage of this approach is the near-net-shaped production of magnets with more complex geometries. Energy densities of these kinds of magnets are, of course, much lower than those of sintered magnets: the permanent magnet material is “diluted” by the polymer, and the standard bonded magnets are isotropic, which means that the Nd–Fe–B grains in the polycrystalline particles are not textured.
There are two ways to induce texture in a polymer-bonded magnet: (1) using melt-spun flake materials that underwent a hot deformation process (see below); or (2) using materials processed in a dynamic hydrogenation disproportionation desorption and recombination (d-HDDR) process [36]. In the d-HDDR process, larger single-grain particles obtained by strip casting and hydrogen decrepitation (see above) are disproportionated at 800–900 °C hydrogen pressures of up to 100 kPa to form anisotropic, polycrystalline particles in the nanoscale. The dynamic control of hydrogen pressure and temperature during the process makes it possible to preserve a high degree of texture within the individual grains—the so-called texture memory effect [37]. Finally, it is important to note that HD and HDDR processing constitutes an important recycling pathway for NdFeB magnets [38–41].
Nanocrystalline, melt-spun Nd–Fe–B can also be hot pressed and hot deformed to make isotropic and anisotropic permanent magnets, respectively [42]. By backward extrusion of the compacts, ring-shaped magnets or those with a high degree of texture and radial magnetization can be produced [43]. The great advantage of nanocrystallinity are the high values of coercivity that can be achieved, since grain size correlates negatively with coercivity until the sizes of the grains scale-up to match with those of the critical single-domain size of Nd–Fe–B, that is, at around 300 nm. In addition, nanocrystalline materials are more corrosion resistant than sintered magnets [44]. The disadvantages of hot pressing and compaction are the relatively high processing costs, caused by the noncontinuous nature of the batch-process and expensive pressing tool that have to be changed regularly. In addition, energy densities of hot-deformed magnets are lower than those of sintered magnets, due to a lower degree of texture that can be achieved.
Applications of NdFeB Magnets
According to Constantinides [45], more than 20 types of applications could be identified for rare-earth magnets. Different ways of classifications for REE magnet applications can be found. Figure 5 shows the relative share of REE magnet applications, predicted for the2012 year end by Shaw and Constantinides [23]. HDDs and CD/DVDs used to be the number 1 applications of the REE permanent magnets, and as is seen from the data, motors and generators are, however, becoming the most important applications in recent years. Although these figures are relatively old, and there are quite some discrepancies from different sources and for different years, it is nevertheless possible to observe some general trends. The classification and grouping of applications are sometimes different, and will lead to data inconsistencies. It is now a commonly accepted fact that the market for HDD and CD/DVD drives is shrinking, and that the use of the magnets in the transport sector, in particular EV/HEV and E-bikes, is rapidly growing.
Shares of the different applications in the global NdFeB market for year 2012 [23] (reprinted with permission)
Table 2 lists the main applications of NdFeB magnets and the relative market size and the total estimated annual demand. The total estimated demand for the REE magnets is about 63,000 tons, excluding the household appliances. According to the above incomplete statistics for 2015 in [45], the main application sectors are conventional automobiles (43 %), wind turbines (17 %), computer HDDs (15 %), EVs/HEVs (14 %), and electric bikes (12 %). However, large uncertainties arise from the use of magnet in conventional vehicles. If only the reported incomplete figures for 2015 by Constantinides [45] are used, the total reported six sectors accounted for 37,500 tons, and the shares of the six reported sectors will be wind (23 %), HDD (20 %), HEV/EV (19 %), e-bikes (16 %), speakers (12 %), and air conditioners (10 %). When recycling is concerned, the wide range of life span of different products needs to be carefully considered.
Recycling Potential of NdFeB Permanent Magnets
According to the estimations of Du and Graedel [49], global in-use stocks for four REEs in NdFeB permanent magnets, i.e., neodymium (Nd), praseodymium (Pr), terbium (Tb), and dysprosium (Dy), amount to a total of 97.0 kilotons (kt): 62.6 kt Nd, 15.7 kt Pr, 15.7 kt Dy, and 3.1 kt Tb. These stocks, if efficiently recycled, could provide a valuable supplement to geological stocks as they are almost four times the 2007 annual extraction rate of the individual elements. Different applications of the four REEs as permanent magnets estimated by Du and Graedel [49] are listed in Table 3.
Rademaker et al. [2] estimated the recycling potential of NdFeB permanent magnets for three application sectors by considering the manufacturing and life span of the applications: computer hard disk drives, wind turbines, and automotive industry. The results show that for some time to come, waste flows from permanent magnets will remain small relative to the rapidly growing global REE demand. Policy makers therefore need to be aware that during the next decade, recycling is unlikely to substantially contribute to the global REE supply. In the long term, waste flows will increase sharply and will meet a substantial part of the total demand for these metals. Figure 6 shows the recycling potential of Nd and Dy for the three studied application sectors. Future REE recycling efforts should, therefore, focus on the development of recycling technology and infrastructure. Those authors also emphasized that one of the main challenges for recycling of NdFeB magnets from consumer products is the physical dismantling and up-concentration of small NdFeB magnets in diversified scrap. REE magnets in wind turbines and EV/HEV vehicles are more easily dismantled and physically concentrated, and even reuse of these large magnets is possible after refurbishing. In general, efficient metallurgical separation and refining processes remain the main challenges. It is estimated that approximately 5–10-year period is required to set up a recycling practice.
Predicted recycling potentials of Nd and Dy from EOL permanent magnets for all the 3 sectors (computer hard disk drives, wind turbines, and automotive industry), adapted from Rademaker et al. [2] (reprinted with permission). a Nd2O3 demand for permanent magnets, b Dy2O3 demand for permanent magnets
Guyonnet et al. [50] reported their research on the material flow analysis as applied to REEs in Europe. Based on the analysis of Nd and Dy metal in magnet applications in 2010 in the EU, 1230 tons of Nd flows into the EU to be used for NdFeB magnets, among which at least 597 tons of Nd has been reported into the waste stream (207 tons as landfill and dissipative loss, and 390 tons dispersed in steelmaking) with zero recycling. They indicated that projections for recycled flows around year 2020 for Nd in permanent magnets are on the order of 170–230 tons per year in the EU.
The study of Habib et al. [51] reveals that a normal primary supply is unable to meet the forecasted demand of Nd and Dy in their modeled demand scenarios by 2050. Although recycling is unlikely to close the wide gap between future demand and supply by 2050, in the long term, secondary supply from recycling can meet almost 50 % of the demand, i.e., by 2100. It is evident that recycling can play a major role in reducing the geopolitical aspects of supply risk.
Physical Separation Technologies
Destination of the Permanent Magnets in Different EOL Products
Except for generators and motors in wind turbines and EV/HEVs, the REE permanent magnets are not pre-dismantled, due to their relative small size, from their attached EOL products in computer HDDs and other home electronics such as mobile phones, music players and speakers in the sound systems. The EOL electronic products (e-waste) are generally shredded as a whole after removal of the hazardous components/materials. Due to the strong permanent magnetism, the small magnets will stick to ferrous scrap (steel) after shredding and physical separation using such as magnetic separators, or to a much smaller extent trapped to the nonferrous scrap if the magnets are not liberated and still stuck to the nonferrous components. At present, almost all small permanent magnets used in consumer electronics (at relatively low collection rate), after collection and shredding operation, are lost into ferrous or nonferrous scrap and reported into slag or residues during pyrometallurgical or hydrometallurgical recovery processes of the major metals, which was recently proved by the study of Bandara et al. [52] from the sampled Electric Arc Furnace (EAF) steelmaking slag. EAF steelmaking uses almost 100 % steel scrap as raw materials which may come from various secondary resource, including shredding of EOL vehicles (ELVs) and electronic waste. Their analysis of slag samples indicated that a substantial presence of Nd was detected in the EAF steelmaking slags with an average concentration of 0.032 wt% Nd (320 ppm). The Nd should come from the NdFeB permanent magnets used in small electric motors and acoustic devices in the cars and electrical and electronic appliances.
For the generators in wind turbines, the REE permanent magnets, after reaching their end of first life, can still be manually dismantled and reused or recovered much more easily due to their large size (250–600 kg/MW power). For the conventional automobiles, there are around 40 magnets in small motors and actuators and 20 sensors in a typical automobile, and on average there are about 250 g of NdFeB magnets and 10–20 g of SmCo magnets as stated by Shaw and Constantinides [23]. These small permanent magnets are not pre-dismantled before car shredder, and they are lost to ferrous or nonferrous scrap similar to the magnets in the consumer electronics. The REE permanent magnets in the electric motors EVs and HEVs are relatively larger than in normal cars (on average 1.25 kg, see Table 2) but still difficult to dismantle manually. Depending on the market value, manual dismantling of the REE magnet-containing components in major consumer products could be an option for further mechanical and metallurgical recovery in the future.
Analysis of REE Magnets in the Shredder Material Streams
Conventional Shredding Operation for ELVs and Household Appliances
As is discussed earlier, most of the permanent magnets go to shredders together with the bonded EOL products. A recent study by Bandara et al. [46] gives a good overview about the destinations and concentrations of the permanent magnets and the associated REEs. Their study for shredding ELVs and household appliances indicates that the ferrous scrap of the shredder products contains about 130–290 g of Nd/ton, or 130–290 ppm. Figure 7 illustrates the shredder feed compositions and the destination of the REE magnets for a mixed shredding of ELVs, household appliances, and other sources. This concentration is even much lower than the REEs content in some mine tailings which can be up to 1000–1500 ppm. Without pre-concentration and novel technologies, the low REE content in the scrap is currently very difficult to recover in the metal industry. Technologies for pre-shredder separation are needed for feasible metallurgical recovery.
Illustration of industrial shredding process for typical EOL products and NdFeB magnets in shredder outputs [46] (reprinted with permission)
Magnet Scrap From WEEE Shredding
At the moment, most of the collected WEEEs are shredded without pre-dismantling for the magnets or magnet-containing components such as HDDs from PCs and laptops or speakers from TVs or mobile phones. In principle, the collected WEEEs are directly shredded as a whole after removing batteries or screens, or any toxic/dangerous components and materials. The permanent magnets are normally reported to the ferrous fraction due to its strong magnetics or stick to the shredder systems without separate collection. In this operation mode, the REEs in the permanent magnets have very low concentration and is very difficult to be up-concentrated for economic recovery. Some recycling companies are stockpiling the manually dismantled REE magnet-containing components such as computer HDDs for future REE recovery, since there is no mature or economically feasible REE recover technologies. Shredding of manually dismantled HDDs is normally practiced for data security of data centers and network drives, and the shredded HDDs are sold as ferrous and nonferrous scrap, and thus REEs in the permanent magnet are lost in the subsequent metal recovery in smelters. This will be further discussed in “Shredding and Physical Upgrading of Pre-dismantled Computer HDDs” section.
Habib et al. [53] studied the flow of resources in electronic waste—with a case of EOL computer hard disk drives. Their results demonstrate the complete loss of REEs in the existing shredding-based WEEE treatment processes. Dismantling and separate processing of NdFeB magnets from their end-use products can be a more preferred option over shredding. However, it remains a technological and logistic challenge for the existing system.
There have been several joint industry–academic efforts in developing separate treatment technologies for computer HDDs and compressors of air conditioners, and this will be described in the “Separation of Magnets Prior to Shredding of HDDs and Air Conditioning Units” section following this. Furthermore, within an EU FP7 project REEcover (http://www.reecover.eu), great efforts are being made to develop economically feasible technologies to recover low concentration REEs in the NdFeB permanent magnets from various standard and specific WEEE shredder products. Industrial operation outputs of various product streams (ferrous, nonferrous, and dust) are sampled and characterized systematically, and further upgrading of REE-rich streams (Nd, Pr, and Dy) originating from NdFeB permanent magnets were intensively investigated using various combination of size reduction and physical separation technologies, including thermal demagnetization, screening, cryo-grinding, and sieving. Quite promising results were obtained and reported in a recent conference paper in 2015 [54]. More detailed publications are expected in the near future, according to the project management.
In order to separate the magnets prior to shredding, several problems have to be overcome. First, the magnets are often held in position with glues and the components held in the electronics with screws. Then the magnets are also often coated with Ni or Ni–Cu–Ni, which has to be removed prior to reprocessing. and finally the magnets have to be demagnetized. Ueberschaar et al. [55] suggested that standardized alloy mixtures for NdFeB magnets, their coatings, and alloy types would help to simplify the recovery of the magnet material for a subsequent use. Several solutions have been proposed, and they are outlined below in Separation of Magnets Prior to Shredding of HDDs and Air Conditioning Units” section.
Separation of Magnets Prior to Shredding of HDDs and Air Conditioning Units
Hydrogen decrepitation of Pre-dismantled Computer HDDs
The University of Birmingham (UK) has developed a hydrogen-processing method to extract sintered NdFeB magnets from computer hard disk drives [13, 41]. During this process, either the voice coil assemblies from the hard disk drive or the cropped corner from the drive is exposed to hydrogen at atmospheric pressure and room temperature. The sintered magnets react with the hydrogen forming NdH2.7 at the grain boundaries. The grain boundary reaction is exothermic and results in the Nd2Fe14B matrix grains forming an interstitial hydride. The overall reaction in hydrogen results in about 5 % volume expansion, and as the magnets are brittle, they break apart resulting in friable hydrogenated powder. The coercivity of the powder becomes so low that the hydrogenated material is essentially soft magnetic and is no longer magnetically attracted to ferrous components in the scrap. This process has been named Hydrogen Processing of Magnet Scrap (HPMS) [41]. During the HPMS process, when applied to hard disk drives, the nickel coating peels away from the surface of the magnets as thin sheets. After the HDD components are processed in hydrogen, they are rotated in a porous drum in order to liberate the hydrogenated powder from the electronics. At this stage, the powder still contains small nickel particles. The nickel contamination is subsequently removed by mechanically sieving the powder mixture. Figure 8 shows the pilot facilities developed at the University of Birmingham.
As the hydrogenated powder is much more friable than the nickel flakes, and hence, it mills faster than the nickel, and when passed through a 90-μm sieve, the nickel contamination is reduced from around 2–3 wt% in the starting magnet to below 400 ppm in the final hydrogenated NdFeB powder. This is equivalent to the nickel content of the base alloy, and thus it has entirely been removed. The NdFeB alloy powder which is generated can be used directly to form new NdFeB magnets using a number of routes which are outlined in “Direct Alloy Recycling Routes” section.
Mechanical Separation of Magnets From HDDs and Air Conditioners
The Hitachi Group (Japan) developed a mechanical dismantling and separation technique for NdFeB magnets in HDDs and air conditioners, using a rotational dram. Through vibration and impact by tumbling, the screws fixing the HDD casing become loose, and the magnets can be separated out of the casing in about 30 min. The apparatus can process 100 HDDs per batch and 200 HDDs per hour, much faster than manual dismantling at a rate of 12 units per hour [9, 56, 57]. Hitachi and Mitsubishi Materials have also developed similar technology to recover the NdFeB magnets from the compressors in air conditioners. After cutting off the casing, the rotors in the compressor can be removed and treated by either nonthermal demagnetization using resonance damping demagnetization at room temperature [57] or thermal demagnetization at 400–500 °C. Hitachi may use the similar rotating drum for disintegration of HDDs to loosen the magnets from the air conditioners, and Mitsubishi uses other equipment (e.g., drills). Furthermore, Mitsubishi Materials is also trying to separate the NdFeB magnets from direct drive (DD) motors in washing machines through thermal demagnetization and removing the resin bounding [9]. Figure 9 illustrates the commercial process to separate REE magnets from HDDs and other scraps to automatically separate fragments of precious metals or other valuables [57]. According to Baba et al. [57], trials were to be conducted on a commercial scale. However, no more recent update about the status of commercialization and operation has been reported to the public.
Process used to separate and recover REE magnets from HDDs [57] (reprinted with permission)
Shredding and Physical Upgrading of Pre-dismantled Computer HDDs
Pre-dismantling of computer hard disk drives have been reported by some companies mainly for data security reasons, although this is not a standard practice for normal personal computers from offices and homes. The dismantled hard disk drives are shredded, and normally, the ferrous and nonferrous scraps as well as the printed circuit boards (PCBs) are recovered. In most cases, the REE magnets particles after shredding are not recovered although the REE concentration is much higher than those in the shredded ferrous products from the whole EOL product (e.g., computers).
In the Netherlands, Van Gansewinkel Group (VGG) operates a small shredder for HDD shredding (data destruction). TU Delft (The Netherlands) has recently developed a REE-recovery process from the magnet-concentrated HDD shredder residues together with VGG [58, 59]. It was found that a feasible REE-recovery process is dependent on an efficient liberation and separation of the magnets from the other components of the HDDs, thereby minimizing the amount of bulk contaminations. The study indicates that when a coarse shredder was used for this purpose, magnets were poorly liberated from other components. This generated a lot of magnet-bearing pieces after shredding. Although the typical shape of the magnets or the appearance of scattered dark magnetic particles, enables their selection by visual screening, this collection method is inefficient and not economical, producing large amounts of nonmagnetic materials for further processing. On a contrary, when a fine shredder was applied, magnets were broken into small particles, and the 30-mm steel grate was revealed as an effective tool to retain the magnetic content while still allowing continuous flow of nonmagnetic materials into the outlet stream. Figure 10 shows the grate after HDDs shredding. Even in this case, however, the as-collected grate residue still contains some nonmagnetic metallic pieces. In order to characterize the content of the grate residue, the sample was hand-sorted into nonmagnetic and magnetic materials as illustrated in the upper part of Fig. 9. The total mass ratio of magnet-to-nonmagnet pieces was approximately 2.2. With this approach, approximately 70 % of the magnet particles was collected in the grate for further processing and recovery.
The separately collected magnet residues go through a thermal demagnetization process followed by grinding and screening. After a mild grinding and screening, the magnet concentrate particles of less than 1 mm in diameter represent approximately 63 % of the total collected shredder residue, and a total recovery rate of 95 % has been achieved for all the REEs in the collected residues through physical processing. The concentration of REEs (Nd + Pr) was upgraded from 5 to 7 % in the as-collected fine shredder residues to about 17–20 % after the above-mentioned physical processing. The highly concentrated magnet particles separated from the ductile metal pieces can go through either hydrometallurgical extraction or first processed with slag extraction. The REE product of fluoride or oxide can be produced. Figure 11 illustrates the developed processing flowsheet.
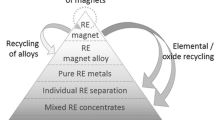
Direct Alloy Recycling Routes
If a clean, nonoxidized form of scrap NdFeB magnets can be separated from the EOL products, in the form of a hydrogenated powder (HPMS), or as a solid magnet, then it is possible to reprocess the material directly from the alloy using a number of routes. It should be noted that these routes are not suitable for shredded material as the contamination levels of this powder will be very high. These direct alloy methods include resintering of the powder, melt spinning, HDDR processing, or recasting back into a master alloy [41]. These routes are used in the primary production of magnets but require some modification in order to handle these secondary materials. For example, a recycled source of sintered NdFeB material will typically contain 2000–5000 ppm oxygen, which is far higher than in a primary cast NdFeB material (typically 300–400 ppm oxygen). Also a recycled source of NdFeB magnets, such as HDD magnets, will contain a range of magnet compositions [60], so questions arise about the consistency of magnetic performance, which can be achieved from magnets made from this material. Despite these potential disadvantages, direct alloy production routes will typically have a smaller environmental footprint compared to recycling routes, which rely on stripping of the REEs (see “Hydrometallurgical Methods and REE Separation by Solvent Extraction” sections). As fewer process steps are required to reprocess directly from the alloy then the cost of magnet production will also invariably be smaller.
Re-sintering of Scrap NdFeB Magnets
Researchers at the University of Birmingham demonstrated that it is possible to produce resintered magnets by processing of NdFeB magnets in hydrogen, blending and milling of the subsequent powder with extra REE material, followed by magnetic alignment of the powder and isostatic pressing to form green compacts. The green compacts were then sintered at ~1080 oC [61, 62]. Further studies by Högberg et al. [63] have shown that it is possible to process solid sintered magnets back into sintered magnets by thermal demagnetization and then milling and sintering, without the use of hydrogen gas. However they reported irreversible losses using this route due to the heating process [63].
Scrap sintered magnets will typically contain 2000–5000 ppm oxygen and most of the oxygen is entrained in the grain boundary phase [64]. On resintering, the grain boundary phase no longer melts due to the higher melting point of the oxide and therefore full density cannot be achieved in the resintered magnets. Therefore this grain boundary phase needs to be replaced. Zakotnik et al. [61] demonstrated that by powder blending 2 at% NdH2.7 into the hydrogenated NdFeB powder that full density could be achieved in the recycled magnets and further studies by Heraiz et al. [65] have shown that by adding >2 at% NdH2.7 the coercivity can be higher than the starting magnet. The remanence of the magnets is typically reduced by around 2–10 % due to the extra nonmagnetic volume of the NdH2.0.7 In subsequent publications REE metals have been added in the form of alloy powders, which has allowed for some tailoring of the magnetic properties. For example, by adding an alloy containing Dy to increase coercivity in a similar fashion to its addition in primary NdFeB magnets [61]. Powder blending in this way is a common technique in the production of primary NdFeB magnets and in a recycled magnet the alloy still supplies extra rare-earth material to aid liquid phase during sintering [66].
Several papers have been published on scaling of the resintering process. For example Zakotnik et al. [67] produced 120 kg of resintered magnets from scrap sintered material which had been pre-separated from the product. However, the variability of remanence, coercivity and maximum energy product are not reported across the entire batch of magnets which were remanufactured. The variability of magnetic performance is as important as the ultimate magnetic performance for the end users of these magnets. Researchers in the EU Framework 7 project REMANENCE (http://www.project-remanence.eu/) have demonstrated that it is possible to extract 5 kg of magnets from ‘real’ scrap hard disk drives provided by Stena Metal in Sweden using the HPMS process, outlined in “Hydrogen Decrepitation of Pre-dismantled Computer HDDs” section, After jet milling, blending, pressing and then resintering the maximum energy product of the final magnets was shown to vary by only ±5 kJ/m3 respectively [68]. This level of variation is similar to that observed in primary magnet production despite the mixed compositional feed which was presented to the process [68].
Hydrogenation Disproportionation Desorption and Recombination (HDDR) of Scrap-Sintered NdFeB Magnets
It was first demonstrated that the HDDR process could be applied to scrap sintered NdFeB magnets in 2004, by Kim et al. [69] and Zakotnik et al. [70], in order to produce isotropic NdFeB powders suitable for processing into resin-bonded magnets. However further research demonstrated that it was possible to produce anisotropic HDDR powdered material [38, 39]. The HDDR process is essentially the same process as that outlined in “Properties and Production Methods of Rare-Earth Permanent Magnet (REPM)” section, However due to the grain size and compositional variation in the secondary material compared to primary cast material, then the temperature and pressure have to be shifted in order to optimize the magnetic performance of the material [40]. The HDDR powder can then be used to produce anisotropic resin-bonded magnets by cold pressing or injection molding in an aligning field [71, 72]. Li et al. [71] demonstrated that it is possible to produce compression molded resin-bonded magnets with a remanence of 0.9 T, a coercivity of 841.4 kA/m, and a maximum energy product of 111.6 kJ/m3. However this work did not address the variability of magnetic performance from ‘real’ mixed scrap feeds. Workers in the EU FP7 project REMANENCE (http://www.project-remanence.eu/) have recently demonstrated the use of HDDR recycled powders for the production of injection molded magnets. An amount of 5 kg of hydrogenated powder was produced using the HPMS process. This powder was then HDDR processed at around 900 °C and 1.2 bar H2 pressure in 400 g batches. The HDDR material was then injection molded with a polyamide binder at Kolektor in Germany in 5 kg batches. It was shown that the coercivity, remanence and maximum energy product only varied by ±1 kA/m, ±2 mT and 0.34 kJ/m3 respectively across the whole 5 kg, which is again in line with magnets produced from primary cast NdFeB material [73].
Re-casting and Melt Spinning of Sintered NdFeB Magnet Scrap
Another potential route to reprocess scrap sintered NdFeB magnets is to remelt the scrap sintered magnets by induction heating, in order to convert the material into a master alloy. The master alloys can then be converted back into magnets using conventional primary processes. This route has the advantage that the oxygen concentration can be reduced in the overall material from 2000 to 5000 ppm in the sintered magnet to around 300-700 ppm in the cast alloy [60]. However this process is typically associated with a yield loss of around 20–30 %. This type of reprocessing is a common way of reprocessing sintered production scrap in the magnet industry. The oxygen is removed in the slag phase, however this does also cause significant wear of the crucibles.
It is also possible to convert sintered magnets into melt-spun ribbons, as in “Properties and Production Methods of Rare-Earth Permanent Magnet (REPM)” section, which are suitable to form resin- bonded magnets. [74]. The magnets are induction melted and then the molten material is ejected under an argon pressure onto a rotating copper wheel to produce ribbons of nanocrystalline NdFeB. This route will suffer from similar yield losses compared to the recasting route for master alloys. Itoh et al. [74] demonstrated this route in 2004 and produced magnets with a remanence of about 0.69 T, a coercivity of about 0.70 MA/m, and a maximum energy product of about 71.0 kJ/m3.
Metallurgical Extraction and Separation
Except for the large NdFeB magnets used in wind turbines and electric motors of EVs/HEVs, direct alloy recycling will be difficult and impractical for the rest of applications. Metallurgical extraction, separation and refining are necessary for small REE magnets and the magnets originated from shredded EOL products and even manually dismantled REE magnets from computer HDDs or other consumer products. In the case of computer HDDs, Ueberschaar et al. [55] conducted a detailed study for the recycling and recovery of REEs and other valuable metals from HDDs, and they emphasized that high variations in alloys used for the voice-coil actuator and for the spindle motor magnet conflict with a direct reuse of the magnets or reutilization of the magnet material for resintering processes. Together with alloy composition changes over time due to fluctuating raw material prices and increased demands for physical properties, eventually hydro- or pyrometallurgical recovery processes are needed, after certain levels of dismantling and physical separation and mechanical processing.
There have been many studies for the recovery of Nd and other associated REEs (Pr, Dy, Tb) through different types of metallurgical processes: hydrometallurgical, pyrometallurgical or electrochemical, or combinations of these techniques. However, most of these studies have focused on the highly concentrated and relatively clean waste magnets of manufacturing scrap or production waste: the new solid scrap and cutting sludge “swarf” [75] which accounts for 20–30 % of the starting alloy [9]. The solid scrap is normally remelted (after coating removal) within the magnet manufacturing plant for direct reuse. However, the swarf requires chemical or metallurgical separation and refining mostly through hydrometallurgical leaching route to produce rare-earth fluorides or oxides as raw materials for conventional metal production (molten salt electrolysis or metallothermic reduction). Recently published comprehensive reviews on rare earths’ recycling by Binnemans et al. [8], Tanaka et al. [9], Takeda and Okabe [10], and Firdaus et al. [11] are good sources of reference, covering large variety of metallurgical methods. Binnemans et al. [8] gave a good comparison for different metallurgical methods as is partially listed in Table 4.
For the small magnets used in the EOL products, little recycling efforts are identified and less than 1 % of the EOL REEs (including magnets) has been recycled according to many expert views, mainly due to the their small size, tight bonds with the product and the multiple type mixture of different magnets (ferrites, SmCo and NdFeB) [9].
As discussed in “Physical Separation Technologies” section, the magnet or REE concentration is too low for economically feasible metallurgical recovery if the EOL products (automobiles, home electrical appliances and consumer electronics) are directly shredded. Pre-dismantling and up-concentration through physical processing are critical for viable chemical or metallurgical recovery. Recovery of the minor amounts of REEs from the magnet scrap is technically and economically challenging. Allocating the REEs in the magnet scrap into the by-product or waste streams via smelting processes of main metals is a potential possibility, including slags, flue dust, solid leach residues and waste solutions. Therefore, a total materials recovery from EOL products (containing REE magnets) would be the direction for future research and development, in parallel with magnet pre-dismantling approach.
Hydrometallurgical Methods
The key steps for hydrometallurgical route are leaching of the magnets or magnet scrap, followed by separation of individual REE species (Nd, Pr, Dy) using solvent extraction, ion exchange, or ionic liquid techniques. Afterward, either selective precipitation of the mixed REEs from co-dissolved non-REE species, or precipitation of individual REE salts or compounds are required, and finally conversion to REE fluorides or oxides could be made. The challenges for the hydrometallurgical process to recover the REEs in the EOL permanent magnets, in particular the REE dilute waste streams, are the selective dissolution, up-concentration and separation of REE species from the major species, and total recovery of all major metals at the same time. Therefore, new separation technologies using novel solvent extractants, ionic liquid, ion exchange resin or the alike, are needed to extract REE species from low concentration leaching solutions.
Leaching of NdFeB Magnet Scrap
Leaching is always the first step to dissolve the REEs in the magnet or magnet scrap, preferably prior to other metals in the magnet and magnet scrap. Depending on the complexity level, different dissolution methods could be used. Dissolution of magnet scrap can be performed in three different ways: (1) complete dissolution of the NdFeB magnet (with or without a prior roasting step), (2) roasting followed by selective leaching of the REEs, and (3) selective conversion of REEs in solid magnet or magnet scrap directly to a new solid phase. Alternatively, transformation of REEs in the magnet scrap into REE compound precipitates could be realized, based on solubility of REE salt at different temperatures or under hydrothermal conditions.
Complete leaching (with/without roasting) H2SO4 leaching at room temperature has been developed by Layman and Palmer in 1993 [17] of US Department of the Interior Bureau of Mines in 1993 for relatively clean production scrap. All components in NdFeB magnets will be dissolved. Nd can be precipitated as double sulfate salt which could be easily converted to NdF3 or Nd2O3. However, large amount of Fe dissolved in the solution needs to be properly disposed (jarosite precipitation). Recently Abrahami et al. [59] successfully applied a similar H2SO4 leaching for the treatment of complex EOL NdFeB magnet scrap from an HDD shredder. Very recently Bandara et al. [76] have demonstrated a HCl leaching process to selectively dissolve NdFeB magnet from shredded electric motors—a mixture of REE magnet, (stainless) steel and copper. The thermally demagnetized magnet particles were completed dissolved in 4 M HCl solution for 24 h at room temperature, while the (stainless) steel and copper particles remained completely unreacted. The leach solution was subsequently treated with oxalic acid to precipitate REEs (Nd, Pr, and Dy) as REE oxalate, and the effluent containing Fe and other dissolved transition metals was suggested conceptually to go through a pyrohydrolysis process to generated iron oxide and HCl back to leaching. A total of 82 % REE recovery has been reached from the total processing from shredding to oxalic precipitation with a product purity of 99.8 % of REE oxalate.
Yoon et al. [77] used an oxidative roasting at 500 °C for sintered scrap and at 700 °C for bonded scrap followed by H2SO4 (2 mol/L) leaching at 50 °C for 2 h and double salt precipitation. Dissolution of Nd2O3 and Fe2O3 seem to be much faster than direct leaching of the magnet, but there is no selectivity of leaching Nd2O3 over Fe2O3. The achieved recovery of Nd yield was over 99.9 %, but about 95 % of iron was also dissolved similar to Layman and Palmer [17]. HCl could also be used for complete magnet dissolution [78]. Subsequently, REEs can be precipitated from leach solutions by adding oxalic acid or hydrogen fluoride to form oxides or fluorides.
Selective leaching (with roasting) Selective Nd leaching could be obtained by first roasting of the magnet in air at 700 °C followed by H2SO4 (4 mol/L) of Nd2O3 by controlling pH at 70 °C for 3 h with pulp density of 100 kg/m3 [79]. However, it reached only 70 % Nd leaching recovery. Formation of NdFeO3 at higher roasting temperatures may hinder the dissolution of Nd. Selective leaching after oxidative roasting at 900 °C for 6 h was also reported by subsequent dissolution with HCl (0.02 mol/L) at 180 °C for 2 h [80, 81]. More than 99 % REE and less than 5 % Fe recovery have been reached.
Hydrothermal conversion Itakura et al. [82] investigated the hydrothermal treatment of Nd–Fe–B sintered magnet using a mixture of hydrochloric and oxalic acids. Hydrothermal treatment is an in situ conversion method to change one mineral to another under hydrothermal conditions (high temperature and high pressure). They used a mixture of HCl (3 mol/L) and oxalic acid (0.2 mol/L) to recover Nd as oxalate. A Nd conversion of over 99 % was achieved at 110 °C for 6 h in the magnet recovered as solid precipitate of Nd2(C2O4)3 with the purity of 99.8 %.
Electrochemical dissolution At TU Delft, a new method using anodic dissolution of NdFeB permanent magnet scrap is under investigation to dissolve REE species electrochemically [83], separating non-REEs in the scrap into anode slimes or precipitated onto the cathode, through accurate control of electrode and cell potentials, current density on the electrodes, and electrolyte chemistry. It is proved technical feasible to dissolve the REEs from the magnet anode, and simultaneously dissolve ferrous metals and reprecipitate them as oxides/hydroxides in the solution or as metals on the cathode.
Leaching methods offer the flexibility of magnet and magnet scrap dissolution. However, selective leaching of REEs in the magnet scrap is challenging due to the presence of large amount of Fe in the magnet alloy and in the scrap. Quite often a high temperature treatment is needed to increase the selectivity of REEs over Fe and other transition metals. For more detailed description and more leaching methods applied to NdFeB magnet scrap, please see the recent reviews by Binnemans et al. [8] and Tanaka et al. [9].
REE Separation Through Solvent Extraction
Solvent-extraction separation of ions from an aqueous solution is based on the formation of complexes between ligand molecules soluble in an organic phase and ions present in an aqueous phase and the transfer of these complexes to the immiscible organic phase. The distribution ratio (D) represents the distribution of the ion of interest between the two immiscible phases. It is defined as the ratio of the total concentration of the ion/element A in the organic phase and the total concentration of A in the aqueous phase (D A = [A]org/[A]aq) The separation factor (SF), defined as SFA/B = D A/D B, is used to show the degree of separation between two solutes A and B in an extraction system. It is important to understand that the distribution ratio of a metal depends both on the chemical equilibria between the different forms of the metal in both phases and on the Gibbs free energy change which occurs when a metal containing species is transferred from one phase to the other. The efficiency of solvent-extraction processes depends on the chemical properties of the ions to be separated, the properties of the ligands and the solvent that they are present in (often called the diluent), the pH of the aqueous phase, the temperature, and other parameters, such as the efficiency of mixing between the aqueous phase and the organic phase and the time of contact between these phases.
The body of literature on specific solvent-extraction processes and extractant systems for separation of REEs is very large. Separation of lanthanides from actinides has been of major interest in the nuclear industry where it is necessary to separate the lanthanides from the actinides. This has inspired to a very large research effort since the 1950s. One of the difficulties in separating the REEs, or lanthanides, by methods like solvent extraction is caused by the fact that they are closely related to each other based on their chemical properties [84]. They are located in the f-block in the periodic table and due to the consecutive filling of 4f-orbitals going from Ce toward Lu, while having the same number of 6 s electrons, the properties of these elements are very similar even though the shielding of the increased positive charge from the nucleus by the electrons create differences in atom radii (“the lanthanide contraction”). The oxidation state +3 is the most common one, both in solid compounds and in solutions. One result of the similarity is that the lanthanides occur together in ores. The similarities in thermodynamic properties influences the formation of complexes in aqueous and organic phases and thus the efficiency of solvent-extraction separation systems. Small differences in the shielding of the charge of the nucleus by 4f electrons between lanthanide ions have to be used to create systems for separation of these ions from each other or in groups [85, 86]. In the production of pure REEs from virgin ores containing mixtures of them with additional elements this generally means that the solvent-extraction separation process must include many mixer settler steps in order to give a sufficient purity of the products. Recycling of REEs from spent products, such as permanent magnets, by leaching and solvent extraction presents an interesting opportunity since the REEs present in the system have been used as a group and can be recovered and reused as a similar group. However, if the recycling is supposed to produce pure REEs full separation needs to be done.
In industrial solvent-extraction separation of REEs the most common ligands are organophosphorous compounds, such as the di(2-ethylhexyl)-phosphoric acid called D2EHPA [87]. Typical separation factors between adjacent REEs using DEHPA is around 2.5 [88]. Separations of REEs from solutions based on different acids by DEHPA have been investigated by several groups showing that aqueous phases based on hydrochloric and sulfuric acids give better results than those based on nitric acid [89–91]. Other organophosphorous ligands that have attracted a lot of interest for separation of lanthanides are saponified 2-ethyl-hexyl phosphonic mono-2-ethyl-hexyl ester (EHEPA or PC88A) and bis(2,4,4-trimethylpentyl) phosphinic acid (Cyanex 272) [92]. EHEPA was recently compared with D2EHPA for separation of Nd, Dy, and Y from hydrochloric acid solution by Mohammadi and co-workers [G]. The results showed that both ligands separately and in mixtures extracted the metals in the order of preference Y(III) > Dy(III) > Nd(III). Both ligands are acidic, which means that H+-ions are released in the formation of REE-ligand complexes. This introduces the pH of the aqueous phase as a factor that needs to be controlled when controlling a process. Depending on the concentration of REE ions in the solution pure D2EHPA or a mixture of D2EHPA and EHEPA gave the best extraction efficiencies. Different complex stoichiometry dominates depending on the relation between REE and ligand concentrations in the respective phases. The best separation between Nd and Dy was achieved using pure D2EHPA.
A rather detailed review about alternative solvent-extraction routes for separation of REEs from permanent magnets has recently been published by Yoon and co-workers [92]. The group of extractants most used in industry are the organophosphorous compounds di-(2-ethylhexyl)phosphoric acid (D2EHPA), saponified 2-ethyl-hexyl phosphonic mono-2-ethyl-hexyl ester (PC88A) and bis (2,4,4-trimethylpentyl) phosphinic acid (Cyanex 272). It has, for example, been reported that the extraction capability for REEs by organophosphorus extractants decreases in the order D2EHPA > PC88A (EHEPA) > Cyanex 272. However, the separation factors between adjacent REEs have been shown to vary according to Cyanex 272 > PC88A > DEHPA [93]. Although Cyanex 272 gives the highest separation factor between Pr and Nd of the three extractants compared by Banda and co-workers, the total extraction percentage of REEs by Cyanex 272 was low. Therefore, in order to obtain high separation factor together with high extraction efficiency, solvent-extraction experiments separating Nd and Pr from chloride solution containing both metals in a mixture have been performed by Liu and co-workers using mixtures of Cyanex 272 and tertiary amines [94]. A combination of Cyanex 272 and Alamine 336 gave the best synergy. Although the separation factors of the two metals were not increased, the extraction of both metals was enhanced. Organophosphorous extractants and amines have been compared as single extractants by for example Abreu and Morais [95] showing that the organophosphorous compounds were the most efficient. The conclusion from the review of Yoon and co-workers [92] was that DEHPA and PC88A are the most effective and promising extractants for the separations needed for recycling of magnet REEs with PC88A seen as the most promising. In both cases, kerosene can preferably be used as diluent. A hydrometallurgical process for the recycling of NdFeB magnet waste with focus on the separation of Tb and Dy from Pr and Nd by solvent extraction with PC-88A was recently presented by a group in Germany [96]. Results from tests in a laboratory size mixer–settler plant showed that twelve stages are necessary for nearly complete separation (4 N purity, rare-earth element basis). The authors state that although further optimization can be done, the process already shows significant advantages over the primary production route used in industry. The fact that the recent laboratory investigation of these ligands by Mohammadi and co-workers came to the conclusion that DEHPA is superior to PC88A [89] shows that these systems are complex and that many factors influence the results.
One example of an amide compound that has been shown to be valuable as a ligand or co-extractant in sovent-extraction systems for REE separation is N,N,N′,N′-tetraoctyl-diglycolamide, also called TODGA. TODGA is a solvating extractant, which creates strong tridentate complexes with metal ions and has shown particularly good extraction properties for REEs ions in terms of selectivity in comparison to other ions in the aqueous solution [97]. This extractant has shown good stability at room temperature and miscibility with the commonly used diluents. A disadvantage for large-scale industrial use is currently the price of this reagent.
The recycling of metals from spent products presents new challenges in the form of dilute flows of REEs mixed with other elements that are not present in the ores used for primary production. Therefore, new separation technologies such as novel extractants for solvent-extraction separations are needed to extract REE species from low concentration leaching solutions and to produce REEs in pure compounds. As stated by Tekda and Okabe in a review on the current status of resource and recycling technologies for rare earths from 2014: “It is (also) important to establish a recycling-based society from the perspective of the conservation of finite and valuable mineral resources and the reduction of the environmental load associated with mining and smelting” [10]. In that context, the chemistry of the ligands used in the solvent-extraction processes for REE production and recycling needs to be taken into account. If possible, it would be beneficial to use ligands based on combustible (leaving no ash products) ligands such as those designed according to the CHON-principle (ligands containing only the elements C, H, O and N) instead of phosphorous or sulfur containing ones. For some time a considerable effort has been put into design and synthesis of efficient reagents complying with the ‘CHON’ principle for the solvent extraction of trivalent f-block elements [98]. An important advance has been the development of malonamides as extractants for lanthanides and actinides. The comparatively easy synthesis of the malonamides, their selectivity for trivalent f-block metals and the fact that they are built according to the ‘CHON’ principle has contributed to, for example, the DIAMEX process [99]. One disadvantage of the malonamides, however, is that the distribution coefficients (ratios) of f-block metal ions are not very high [100]. By increasing the distribution ratio during the extraction part of a purification process, the number of stages of countercurrent extraction required can be reduced thus reducing the size and cost of the plant. Novel extractants for magnet REE solvent-extraction separation are presently being developed at Chalmers University in Gothenburg (Sweden) as a part of the efforts toward better lanthanide recycling options. Three polyamides (multicoordinate, neutral ligands) bearing tetrabutylmalonamide functional groups have been synthesized and evaluated for use as reagents for the solvent extraction of rare-earth elements from nitrate media. These extractants are based on the CHON chemistry, avoiding phosphorous and other ash forming elements which makes it easier to combust the extractant and the diluent once it can no longer be recycled into the process. The results showed good effects of structural modifications of tetrabutylmalonamide on the extraction of 152Eu, which was used as a model for lanthanides due to that it allows for precise radio-analytical (radiotracer) determination. The best distribution ratios for Eu were obtained with the tetraamide 2,2′-(1,2-phenylenebis(methylene))bis(N,N,N′,N′-tetrabutylmalonamide). As for other malonamides a diluent with quite high polarity is favorable for solvent extraction of rare-earth metals [101]. It is important to note that oxidation in supercritical water has been shown to be able to process tributyl phosphate, so in the event that a CHON-extraction system for the recycling of the lanthanides cannot be found then an industrial scale recycling process will still be able to operate without recourse to the incineration of phosphorus rich organic wastes. However the absence of phosphorus and sulfur in the organic waste produced by a solvent-extraction plant would increase the value of such a waste as a fuel.
In some cases, it may be beneficial to have a stationary phase containing the complexation ligands. One example is presented in the work by Kim et al. [102], which reported in 2015, an application of membrane-assisted solvent extraction (MSX) to recover Nd, Pr, and Dy from commercial NdFeB magnets and industrial scrap magnets. A hollow hydrophobic polypropylene fiber membrane module system was used to extract REEs in a single step with the feed and strip solutions circulating continuously through the MSX system. The pores of hollow fibers in the membrane modules were filled with organic phase consisting of the TODGA, Isopar L (An alphatic diluent), and TBP in the ratio of 3:4:3, respectively. The shell side of hollow fibers was supplied with the aqueous strip solution (0.2 M HNO3), and the lumen side of the hollow fibers was fed with the REE-containing solution (1000–2000 ppm) in 6 M HNO3. They found that a multimembrane module configuration with REEs dissolved in HNO3 solutions showed high selectivity for REE extraction with no co-extraction of non-REEs, while the use of HCl solution resulted in co-extraction of non-REEs. REE oxides in highly pure form were produced from the strip solutions. It seems that the application of membrane-assisted solvent extraction to the REE recovery from the scrap magnets would result in a more environmentally friendly and cost-effective process compared with the conventional routes such as precipitation and solvent extraction.
REE Separation by Ionic Liquids
The replacement of the organic phase in solvent-extraction processes is a novel approach in hydrometallurgy. Ionic liquids are solvents that consist entirely of ions. They are nonvolatile and nonflammable so that they can be safer alternatives for molecular organic solvents in extraction processes, including processes for the separation of rare earths [103–107]. The very high concentration of extractants in the case of using undiluted ionic liquids that act as basic extractants (anion exchangers) can lead to very high loadings of metals in the ionic liquid phase and thus to process intensification (less solvent has to be used). The mechanism of solvent extraction with ionic liquids can be different from solvent extraction with molecular solvents, and this offers an opportunity for the development of new more selective separation processes. There are several challenges to deal with when designing ionic-liquid-based solvent-extraction processes: (1) the high viscosity of the ionic liquids phases which hampers mass transport; (2) the tendency of the ionic liquid to extract via an ion exchange mechanism, resulting in losses of the ionic liquid components to the aqueous phase; (3) the hydrolytic instability of some fluorinated anions such as hexafluorophosphate; (4) the recyclability of ionic liquids; (5) (cyto)toxicity issues; (6) the high price of most types of ionic liquids. Examples of REE separation systems relevant to magnets recycling making use of combinations of ionic liquids and extractants are (1) 1-methyl-3-octyl imidazolium bis(trifluoromethylsulfonyl)imide [C8mim][Tf2N] in combination with N,N-dioctyldiglycolamid acid (DODGAA) [105] and (2) 1-alkyl-3-methylimidazolium hexafluorophosphate [Cnmim][PF6] (n = 2,4) in combination with D2EHPA, which is one of the most frequently used extractants for conventional solvent extraction of REEs [108]. DODGAA is a modification of the extractant TODGA which has proven to have a high affinity to lanthanides. In systems with the ionizable DODGAA the REE ions are extracted as 1:3 complexes and the metal ions can be completely stripped by acid solution. This is a significant improvement since the stripping has been a troublesome issue for many systems based on ionic liquids, such as TODGA [109–111]. Kikuchi et al. [112] investigated the extraction of the REE ions Pr(III), Nd(III) and Dy(III) with tributylphosphate (TBP) in the ionic liquid tricaprylmethylammonium nitrate [A336][NO3].
During the recent years, the Binnemans group at KU Leuven (Belgium) has made major research efforts to develop sustainable ionic liquid processes for the recovery of REEs from end-of-life NdFeB magnets. The focus of this work is on the use of hydrophobic ionic liquids with nonfluorinated anions (chloride or nitrate). By increasing the alkyl chain lengths on the cation, the ionic liquids can be made hydrophobic (immiscible with water) without the need of using hydrophobic fluorinated anions. The ionic liquids are typically quaternary ammonium of phosphonium ionic liquids such as Aliquat® 336 or trihexyl(tetradecyl)phosphonium chloride (Cyphos® IL 101). In case the metal ions are extracted via an anion exchange mechanism, the loss of chloride or nitrate ions from the ionic liquid phase to the organic phase is not a major issue. The ionic liquids are used in undiluted form, i.e., without addition of molecular diluents, to take full advantage of the ionic liquid properties. The selectivity of the extraction process can be tuned by the choice of the ionic liquid anion. For instance, transition metal ions are extracted from a chloride aqueous solution to a chloride ionic liquid phase leaving the rare-earth ions behind in the aqueous solution [113], while the reverse situation holds for extraction from a nitrate aqueous phase to a nitrate ionic liquid [114]. After removal of the transition metals by extraction with a chloride ionic liquid, the REEs can be recovered from the raffinate by precipitation with oxalic acid, followed by calcination of the oxalates to oxides [115]. An efficient process was developed to extract Nd and Dy from a nitrate solution, leaving Co behind, followed by separation of Nd/Dy by selective stripping with EDTA [116]. Dupont and Binnemans [117] developed a new recycling process for (microwave) roasted NdFeB magnets, based on the carboxyl-functionalized ionic liquid betainium bis(trifluoromethylsulfonyl)imide, [Hbet][Tf2N]. This ionic liquid shows a temperature-dependent miscibility with water (=thermomorphic behavior): the [Hbet][Tf2N]–H2O system is biphasic below 56 °C, but it forms one single-phase below this temperature. Using the thermomorphic properties of the [Hbet][Tf2N]–H2O system, a combined leaching/extraction step was designed. The change from a homogeneous system during leaching (80 °C) to a biphasic system at room temperature causes the dissolved metal ions to distribute themselves among the two phases: Fe is reported to be present in the ionic liquid phase, while Nd, Dy, and Co remain in the aqueous phase. By selective stripping and precipitation steps, the REEs could be recovered as a mixture of Nd2O3/Dy2O3 (to be separated in a separate process), cobalt is recovered as CoO, and the ionic liquid [Hbet][Tf2N] could be regenerated for reuse.
Pyrometallurgical Methods
The main purpose of high-temperature processing of NdFeB magnet scrap is to selectively convert REEs in the magnet into another phase which separates the main non-REE components. The separated REEs in the other phase is more concentrated and can be used for RE metal production through molten salt electrolysis or metallothermic reduction. The identified pyrometallurgical extraction methods can be divided into the following groups: (1) roasting, (2) liquid metal extraction, (3) molten salt extraction, (4) molten slag extraction, and (5) electrochemical processing. As can be seen from the discussions below, most of the pyrometallurgical methods are applicable to only highly concentrated magnet scrap (production waste: off-specs and swarf), and adaptation is required to treat the dilute EOL magnet scrap. Slag extraction is a promising method to separate and up-concentrate the REEs in the low concentration magnet-bearing scrap such as shredder residues, however, subsequent hydrometallurgical process steps such as REE leaching are needed. More detailed description on high-temperature recovery from NdFeB magnet wastes can be found in a few recent reviews [8–11]. Firdaus et al. [11] emphasized that high-temperature processing is one alternative that can be used to avoid large consumption of water and production of hazardous waste in the recovery process. A number of techniques are available for recovering REEs from magnet waste by high-temperature processing, but all are still at the research stage, and currently none has been applied on a commercial scale in industry. It was also indicated that combinations of methods may be required to completely recover REEs from magnet waste.
Roasting
The aim of roasting is to change the form or state of REEs in the NdFeB magnets at high temperatures, so that the roast product could be more selectively dissolved hydrometallurgically for REEs, leaving Fe and other constituents in the magnet and the scrap stream in the solid residue. After roasting, a more effective separation could be reached in the subsequent hydrometallurgical processing.
Chlorination roasting For selective chlorination of REEs in the magnet, various chlorination agents have been investigated mainly by the Japanese researchers: using metal halides such as MgCl2 to convert the REEs into molten NdCl3 and DyCl3 at 1000 °C [118], using FeCl2 at 800 °C [14], using NH4Cl at much lower temperature of 300 °C [15], or using Cl2 gas and AlCl3 (the so-called chemical vapor transport process) [119]. In most of these cases, the RECl3 (NdCl3 and DyCl3) were distilled at elevated temperatures to separate from the Fe-based residue. For all chlorination approaches, selectivity and reaction rates are key issues, in particular when treating highly contaminated and partially oxidized magnet scrap.
Sulfation roasting During sulfation roasting, the metallic elements are sulfated to form water-soluble sulfates, preferably for REE only, so that the non-REEs such as iron will be restricted at the solid state in oxide form. There are two steps for this process [120]: in the first step, the whole magnetic material is converted into sulfates by digestion in concentrated H2SO4 at room temperature; in the second step, the resulting mixture is roasted at a temperature at which the REE sulfates are still thermally stable, but the iron sulfates decompose into iron oxide insoluble in water.
Taking advantage of the difference in temperature stability of REE sulfates and iron sulfates, the process of sulfation roasting, followed by water leaching allows the separation of more than 95 % of REE in the leaching solution with no iron present. Iron remains in the leaching residue forming a marketable hematite-dominated by-product. This hematite (Fe2O3) could be used as a red pigment. Impurities such as Co and Ni were also reported to be present in the residue, thus enabling the production of a leaching liquid with at least 98 % REE purity. SO3 gas released by thermal decomposition of the iron sulfates can be recovered and used for production of H2SO4. In this way, a cyclic process can be developed, minimizing emission of SO3.
Oxidation roasting The objective of oxidation roasting is to convert the REE magnet into oxides for all metallic elements, and the subsequent leaching process of REEs becomes easier or faster, or more selective. The oxidation roasting—REE leaching has been studied by many researchers [77–81], and it has been discussed in the previous section for hydrometallurgical leaching process. It is important to combine the leaching selectivity and roasting conditions.
Selective Extraction of REEs Through Melt Processing
In this type of method, magnet scrap is treated with either a molten metal, a molten salt, or a molten oxide slag. The REEs in the magnet will be selectively converted or dissolved to another phase, leaving the iron, boron and other contaminating metals as unreacted solid or as a new molten ferrous alloy. This leads to the separation of REEs from the rest of the magnet and magnet scrap.
Liquid metal extraction The Ames Laboratory (USA) has developed a liquid metal-extraction process to selectively recover REEs from NdFeB and other REE-bearing alloys using Mg as extractant [121], and later the process was studied further by other researchers, [16, 122, 123]. In this process, the molten Mg and potentially other alkaline earth metals (Ca or Ba) claimed in Ref. [121] can dissolve selectively Nd and other REEs such as Pr and Dy at about 1000 °C (above the melting point of the extractant metal), leaving Fe and B or other transition metal unreacted in solid state. The formed molten Mg–REE alloy could be separated from the iron and other transition metals (in solid state). The Mg–REE separation can be realized through distillation to evaporate Mg metal due to its high volatility. However, this process is very slow (24–72 h), and it cannot be applied to (partly) oxidized NdFeB scrap. Alternatively, selective extraction of Nd was also reported successfully using silver [124] and copper [125]. Using Ag as the extractant, a Nd-Ag alloy containing 45–50 at % Nd was obtained in the temperature range of 1000–1300 °C, and Nd could be separated as Nd2O3 from silver by oxidation of the Nd-Ag melt in the air [124]. According to Moore et al. [125], after a quick arc or induction melting of the magnet–copper mixture (holding time 1–2 min.), a Cu-Nd rich intermetallics phase was formed upon slow cooling 350 K/min.) on the outer shell of the ingot, when a higher fraction of copper was used in the mixture feed (Cu > 21.5 %, in weight percentage unless otherwise stated). The bath temperature of arc melting could not be detected and controlled, but the induction melting temperature was reported to be in the range of 1450–1500 °C. The Nd–Cu-rich shell (nonmagnetic) is brittle and was mechanically fragmented, thus a mechanical separation from the ferromagnetic core (Fe-rich) could be obtained with magnetic separation. However, the separation is not very efficient: ferromagnetic fraction still contains about 10.4 % Nd and 7.8 % Cu, and nonmagnetic fraction contains 11.4 % Fe and 43.9 % Cu. Furthermore, further Nd–Cu separation has not been investigated, and the alloy contains almost equal amount of Nd and Cu of 44 %. This could be a fundamental limitation and challenge by using copper as an extractant.
Molten salt extraction At Anhui University of Technology (China), Hua et al. [126] reported an integrated process using molten chloride salt (MgCl–KCl) to selectively dissolve Nd and other REEs in the magnet scrap at temperatures of 700–1000 °C with an REE recovery yield of approximately 90 %. NdCl3 in molten the MgCl2–KCl salt mixture can be subsequently electrolyzed to produce a magnesium-neodymium alloy directly. Tanaka et al. [9, 127] proposed a process based on molten fluoride to remove the oxidized rare earths in the NdFeB magnet scrap. In this process, the molten fluoride reacts with the rare-earth oxides (REOs) and the formed REO–fluoride mixture can be used in molten salt electrolysis for REE metal production. The nonoxidized NdFeB scrap does not react, and it obtained as a cleaned master alloy that can be used for the production of new NdFeB magnets. It should be noted that the molten chloride and molten fluoride processes are complementary: molten chloride salts can be used for treatment of nonoxidized NdFeB magnet scrap only, while molten fluoride salts are suitable for the treatment of oxidized NdFeB magnet scrap only. Hua et al. [128] also reported the REE magnet treatment using a fluoride melt, and REEs in NdFeB magnet scrap have been successfully recovered using AlF3–NaF melts as the fluorination reagent. Furthermore, active research is going on at TU Delft as part of EU FP7 funded project EREAN (http://www.erean.eu) to investigate the salt extraction of NdFeB magnet scrap for further molten salt electro-deposition of the REEs.
Molten slag extraction Two different slag treatment methods have been reported. In the glass slag method, the REE alloy is brought into contact with molten B2O3 that is able to selectively oxidize and dissolve the REEs from the NdFeB alloys to form Nd2O3–B2O3 slag [19]. The slag can be then leached with acid to dissolve Nd and the Nd can be recovered from the leachate by precipitation as Nd(OH)3. However, the yield of Nd recovery is relatively poor [129]. The second slag method is the use of CaO–SiO2–Al2O3 or CaO–CaF2 fluxes to extract the REEs in the complex magnet scrap at about 1500 °C [130]. This method is very effective for EOL magnet scrap such as HDD shredder residues with more than 99 % REE recovery. It can separate completely the REEs in the magnet from the ferrous materials in the NdFeB magnet and the mixed scrap. The REE-rich slag can be used for hydrometallurgical extraction, and the separated iron-rich alloy can be a good source of raw materials for steelmaking. The advantages of molten slag extraction are its capability to effectively separate REEs and iron, and the recovery of both fractions at the same time. According to Tanaka et al. [9], a Japanese group reported an in-plant waste (sludge) separation process where the magnet waste separates into liquid iron and solid rare-earth oxide phases at 1550 °C under argon atmosphere with a low oxygen partial pressure without any flux addition. It was found that all of the REEs in the magnet waste were converted to the oxide phase, and the formed REOs were converted to RE metal through conventional technologies.
Direct melting Recently a research group at Shanghai University reported a new method for the treatment of relatively clean NdFeB magnet (without Ni coating) [131]. They first melted the magnet in a vacuum induction furnace at about 1400 °C in a graphite crucible, after which the NdFeB magnet will form REE carbides in the form of NdFeBC alloy. The solidified carbon-saturated alloy containing REE carbides are milled to fine particles and treated with water at room temperature for selective hydrolysis, and the NdC2 in the alloy phase was converted to Nd(OH)3, and the mixed metallic iron and iron carbide are removed by magnet separation. The recovered Nd(OH)3 is easily calcined to Nd2O3 for direct commercial use or as raw materials for production of Nd metal through molten salt electrolysis. The process of vacuum induction melting (VIM) followed by hydrolysis and magnetic separation (HMS) was named as VIM–HMS method by the authors. This is a very clean process, and no toxic chemicals are used and no significant solid residues or effluent are generated. However, the method was developed with very clean magnets, and it is not clear if the method is suitable for EOL magnet scrap with low rare-earth content and highly complex compositions. Contamination of other transition metals in the NdFeB magnet-bearing streams such as copper and nickel may be a challenge.
In the EU FP7-funded project REEcover (http://www.reecover.eu), an alternative pyrometallurgical treatment of a REE-containing ferrous fraction from physically upgraded WEEE shredder products was reported recently [132]. The WEEE shredder product was first thermal demagnetized, and a fine fraction (after screening) was ground and screened to 75 μm [54]. The fraction above 75 μm is used for pyrometallurgical treatment, and the fraction below 75 μm can be treated directly with hydrometallurgical leaching. The upgraded ferrous stream from the shredder product contains about 6500 ppm REEs, originating from NdFeB permanent magnet (Nd, Pr, and Dy). It was melted at 1600 °C under reducing conditions in the graphite crucible for one-hour holding time. A complete separation of REEs from the ferrous fraction was achieved, and all REEs in the scrap were oxidized into a slag phase. The amount of the resulted slag is small, but with a relatively high concentration of REEs (over 33 % in REO form). The REE-bearing slag contains also Al2O3, SrO, BaO, SiO2, and CaO, with a melting temperature in the range of 1320–1400 °C. The REE-concentrated slag is used for REE separation and extraction through hydrometallurgical leaching and further precipitation to produce more pure REOs, and finally for producing REE metal or alloy through molten salt electrolysis. More research is under way to optimize the melting conditions, e.g., using fluxing agent for enhanced REEs slag formation, to achieve more favorable and selective leaching recovery of REEs. However, the resulting concentration of REEs in the slag will be much lower than the above reported figures.
Direct Electrochemical Refining
Electrochemical processes can be applied to the metallic magnet scrap based on electro-refining principles in the molten salt [9, 133]. In a molten salt electro-refining cell, the impure REE magnet scrap is used as starting anode, and the REEs (Nd and/or Dy) can be selectively dissolved in a molten chloride or fluoride electrolyte, whereas on the cathode, only REEs are preferably deposited forming Nd-based alloys. In the cell, a REE—an iron group metal alloy is used as bipolar electrode (diaphragm).
High-temperature processing of NdFeB magnet scrap has also been reviewed by Takeda and Okabe [10], and they described advantages of the so-called dry process over the hydrometallurgical “wet processes.” They predicted that the EOL scrap from products is expected to be generated in the future at a constant volume such as the large magnets in EVs and HEVs. Therefore, efficient mechanisms and processes for recovering Nd and Dy from these types of product scrap should be developed for the future needs. Firdaus et al. [11] indicated a number of challenges for REE recovery from NdFeB magnet scrap: (1) the different mix of wastes produced—the complexity of magnet waste composition; (2) the effects of contaminants on the recycling process; (3) optimization for mutual separation of REEs (Nd, Dy, Pr); and (4) economic feasibility of the recycling and recovery process and lifecycle of the magnet-containing products (i.e., magnet scrap availability).
Life Cycle Assessments
The environmental impact of the life cycle of the REEs used in specific products like magnets can be studied with conventional LCA, the so-called attributional LCA—see, e.g., Finnveden et al. [134]. LCA is a well-developed and acknowledged methodology backed by obligatory ISO Standards 14040 and 14044. The partners of the project MORE, a German national-funded project, addressed the recycling of electric drive motors from the automotive sector [135]. Within the project, the reuse of NdFeB magnets, reuse of the magnetic alloy as well as REE recovery were investigated. LCA results are available for all these three different routes [136]. In this article, only an excerpt of the LCA results for the raw material route is presented as it was identified as the most promising approach. The MORE LCA was conducted according to ISO 14040/44 including a critical review by an independent external expert and is based on small pilot-scale data. Figure 12 shows the system boundaries of the LCA for the recovery of pure neodymium and dysprosium oxides from magnets used in electric motors via a final solvent-extraction step.

Adapted from Walachowicz et al. [136]
System boundaries of the MORE LCA (recovery of pure neodymium and dysprosium oxides)
For the different LCAs conducted for the different MORE process routes, the partners agreed upon the generic composition of a typical NdFeB magnet given in Table 5.
The functional unit for this LCA is defined as recovery of 1 kg REE oxide (Nd: Dy ratio = 2:1). It should be highlighted that for the credit calculation for the recovered REE, a mass allocation as well as an economic allocation was used (see data in Table 6).
Figure 13 below demonstrates the LCA results for the recovery of pure Nd2O3 and Dy2O3 compared to the primary production route. The necessary additional step from REE oxide to REE metal was taken into account for this calculation.

Adapted from Walachowicz et al. [136]
Global warming potential, recovery of pure neodymium and dysprosium oxides (solvent-extraction route)
Also the results for all other environmental impact categories show net benefits. Therefore, the overall recycling process seems to be very promising from an ecological point of view in comparison to the primary production of REEs. However, the good environmental performance should be verified in future LCA studies if further scale-up takes place especially considering that the collection of comprehensive LCA data for the primary routes of rare earths is a pending and challenging task. Sprecher et al. [137] compared the environmental impact of the primary production process of 1 kg of NdFeB permanent magnets with two alternative recycling processes. This work focused on the recycling of NdFeB magnets from HDDs (hard disk drives). The difference of the two recycling processes is the pretreatment step for the HDDs from EOL computers (manual dismantling of the NdFeB magnets vs. shredding of the HDDs). Again the recycling processes show an improvement over primary production of NdFeB magnets from an environmental point of view. It should be noted that the recycling process with the manual dismantling step shows superior results compared with the alternative which includes shredding of the HDDs. The independent results of the MORE partners [135, 136] and other researchers [137] confirmed the ecological advantages of recycling approaches for NdFeB magnets compared with primary production.
Concluding Remarks
This review paper addresses the recycling potential of NdFeB permanent magnets and the identified physical and metallurgical separation and recovery technologies. It is indicated that the annual consumption and demand of the REE magnets are continuously increasing, and the recycling of the REEs from the EOL permanent magnets will be complementary to the supply shortage in the long run. REE recovery from the manufacturing scrap and waste (swarf) of the NdFeB magnets is already a good industrial practice with mature technologies.
However, recycling and recovery of REEs (Nd, Pr, Dy, Tb) from EOL magnets are challenging due to their relatively small sizes used in consumer electrical and electronics, as well as in the conventional automotive industry. Shredding without pre-dismantling of the EOL consumer products will pose great difficulties for up-concentration of the permanent magnets. Development of advanced and low-cost of pre-dissembling technologies or equipment will be crucial for the metallurgical recovery of these critical elements. This has been demonstrated by the developments for computer hard disk drives and air conditioners by Hitachi and Mitsubishi, Birmingham University, Delft University of Technology, and more from WEEE streams in a number of active European initiatives such as EREAN (http://erean.eu), REEcover (http://www.reecover.eu), REE4EU (http://www.ree4eu.eu) consortia and other global government supports in Japan and USA reported in recent years.
There are many different types of metallurgical recovery methods identified from the literature; however, most of them have been studied for relatively clean and highly concentrated new or manufacturing magnet scrap and waste. Adaptation of these technologies to the dilute and complex EOL magnet scrap is very much needed. At the same time, recovery of the major metals and materials in the dilute magnet waste stream (e.g., shredder products) is an important factor for the total success of the permanent magnet and REE recycling. It is believed that no single metallurgical process can be successful for the efficient recovery of the REEs from their EOL secondary resource, and a combination of hydrometallurgical, pyrometallurgical, and/or electrochemical technologies will be the future solution. It is expected that through the globally increasing efforts (Europe, Japan, USA, China) for the research and development, establishment of technologically and economically feasible REE magnet recycling industry could be realized in the near future, if the NdFeB permanent magnet market continue to develop.
References
Jiles D (1998) Introduction to magnetism and magnetic materials, 2nd edn. Chapman & Hall, New York
Rademaker JH, Kleijn R, Yang Y (2013) Recycling as a strategy against rare earth element criticality: a systemic evaluation of the potential yield of NdFeB magnet recycling. Environ Sci Technol 47:10129–10136. doi:10.1021/es305007w
Binnemans K, Jones PT, Van Acker K, Blanpain B, Mishra B, Apelian D (2013) Rare-earth economics: the balance problem. JOM 65:846–848. doi:10.1007/s11837-013-0639-7
Binnemans K, Jones PT (2015) Rare earths and the balance problem. J Sustain Metall 1:29–38. doi:10.1007/s40831-014-0005-1
Elshkaki A, Graedel TE (2014) Dysprosium, the balance problem, and wind power technology. Appl Energy 136:548–559. doi:10.1016/j.apenergy.2014.09.064
Schüler D, Buchert M, Liu R, Dittrich S, Merz C (2011) Study on rare earths and their recycling: final report for the Greens/EFA Group in the European Parliament. Öko-Institute e.V, Darmstad
Moss RL, Tzimas E, Kara H, Willis P, Kooroshy J (2011) Critical metals in strategic energy technologies: assessing rare metals as supply-chain bottlenecks in low carbon energy technologies. Eur Comm JRC Inst Energy Transp. doi:10.2790/35716
Binnemans K, Jones PT, Blanpain B, Van Gerven T, Yang Y, Walton A, Buchert M (2013) Recycling of rare earths: a critical review. J Clean Prod 51:1–22. doi:10.1016/j.jclepro.2012.12.037
Tanaka M, Oki T, Koyama K, Narita H, Oishi T (2013) Chapter 255—recycling of rare earths from scrap. In: Jean-Claude GB, Vitalij KP (eds) Handbook on the physics and chemistry of rare earths. Elsevier, Amsterdam
Takeda O, Okabe TH (2014) Current status on resource and recycling technology for rare earths. Metall Mater Trans E 1A:160–173. doi:10.1007/s40553-014-0016-7
Firdaus M, Rhamdhani MA, Durandet Y, Rankin WJ, McGregor K (2016) Review of high-temperature recovery of rare earth (Nd/Dy) from magnet waste. J Sustain Metall. doi:10.1007/s40831-016-0045-9
Zakotnik M, Harris IR, Williams AJ (2008) Possible methods of recycling NdFeB-type sintered magnets using the HD/degassing process. J Alloys Compd 450:525–531. doi:10.1016/j.jallcom.2007.01.134
Walton A, Williams A (2011) Rare earth recovery. Mater World 19:24–26
Uda T (2002) Recovery of rare earths from magnet sludge by FeCl2. Mater Trans 43:55–62
Itoh M, Miura K, Machida KI (2009) Novel rare earth recovery process on Nd-FeB magnet scrap by selective chlorination using NH4Cl. J Alloys Compd 477:484–487. doi:10.1016/j.jallcom.2008.10.036
Okabe TH, Takeda O, Fukuda K, Umetsu Y (2003) Direct extraction and recovery of neodymium metals from magnet scrap. Mater Trans 44:798–801
Lyman JW, Palmer GR (1993) Recycling of rare earths and iron from NdFeB magnet scrap. High Temp Mater Process 11(1–4):175–187. doi:10.1515/HTMP.1993.11.1-4.175
Wellens S, Thijs B, Binnemans K (2012) An environmentally friendlier approach to hydrometallurgy: highly selective separation of cobalt from nickel by solvent extraction with undiluted phosphonium ionic liquids. Green Chem 14:1657–1665. doi:10.1039/C2GC35246J
Saito T, Sato H, Ozawa S, Yu J, Motegi T (2003) The extraction of Nd from waste NdFeB alloys by the glass slag method. J Alloys and Compd 353:189–193. doi:10.1016/S0925-8388(02)01202-1
USGS (2010-2016) Minerals yearbook, rare earths. http://minerals.usgs.gov/minerals/pubs/commodity/rare_earths/index.html#myb. Accessed 8 May 2016
Goonan T G (2011) Rare earth elements-end use and recyclability. USGS Scientific Investigations Report 2011–5094. https://pubs.usgs.gov/sir/2011/5094/pdf/sir2011-5094.pdf. Accessed 8 May 2016
British Geological Survey (BGS) (2010) rare earth elements. 2010. http://www.MineralsUK.com. Accessed 8 May 2016
Shaw S, Constantinides S (2012) Permanent magnets: the demand for rare earths. Presentation in: 8th International Rare Earths Conference. 13–15 November 2012, Hong Kong. http://roskill.com/wp/wp-content/uploads/2014/11/download-roskills-paper-on-permanent-magnets-the-demand-for-rare-earths.attachment1.pdf. Accessed 8 May 2016
Sagawa M, Fujimura S, Yamamoto H, Matsuura Y, Hiraga K (1984) Permanent magnet materials based on the rare earth-iron-boron tetragonal compounds. IEEE Trans Magn 20(5):1584–1589. doi:10.1109/TMAG.1984.1063214
Alonso E, Sherman AM, Wallington TJ, Everson MP, Field R, Roth R, Kirchain RE (2012) Evaluating rare earth element availability: a case with revolutionary demand from clean technologies. Environ Sci Technol 46:3406–3414. doi:10.1021/es203518d
Kozawa S (2011) Trends and problems in research of permanent magnets for motors-addressing scarcity problem of rare earth elements. Sci Technol Trends: Quart Rev 38:40–54 http://data.nistep.go.jp/dspace/bitstream/11035/2854/1/NISTEP-STT038E−40.pdf. Accessed 8 May 2016
Benecki WT (2013) The permanent magnet market 2015. Presentation in: magnetics 2013 Conference. February 8, 2013 Orlando. http://www.waltbenecki.com/uploads/Magnetics_2013_Benecki_Presentation.pdf. Accessed 8 May 2016
UNEP (2013) Metal recycling: opportunities, limits, infrastructure, contributors. In: Reuter MA, Hudson C, van Schaik A, Heiskanen K, Meskers C, Hagelüken CA (eds). Report of the Working Group on the Global Metal Flows to the International Resource Panel
Gutfleisch O, Willard MA, Brück E, Chen CH, Sankar SG, Liu JP (2011) Magnetic materials and devices for the 21st century: stronger, lighter, and more energy efficient (review). Adv Mater 23:821–842. doi:10.1002/adma.201002180
Kuz’min MD, Skokov KP, Jian H, Radulov I, Gutfleisch O (2014) Towards high-performance permanent magnets without rare earths. J Phys 26:5. doi:10.1088/0953-8984/26/6/064205
Sawatzki S, Dirks A, Dirba I, Schultz Land Gutfleisch O (2014) Coercivity enhancement in hot-pressed Nd-Fe-B permanent magnets by low melting eutectics. J Appl Phys 115:17A705. doi:10.1063/1.4859097
Edström A et al (2015) Effect of doping by 5d elements on magnetic properties of (Fe1 − xCox)2B alloys. Phys Rev B 92:174413. doi:10.1103/PhysRevB.92.174413
Gutfleisch O (2000) Controlling the properties of high density permanent magnetic materials (TOPICAL REVIEW). J Phys D 33:R157–R172. doi:10.1088/0022-3727/33/17/201
Gutfleisch O, Harris IR (1996) Fundamental and practical aspects of the HDDR process. J Phys D 29:2255–2265. doi:10.1088/0022-3727/29/9/006
Woodcock TG, Zhang Y, Hrkac G, Ciuta G, Dempsey NM, Schrefl T, Gutfleisch O, Givord D (2012) Understanding microstructure and coercivity in high performance NdFeB-based magnets (viewpoint paper). Scripta Mat 67:536–541
Gutfleisch O, Khlopkov K, Teresiak A, Müller KH, Drazic G, Mishima C, Honkura Y (2003) Memory of texture during HDDR processing of NdFeB. IEEE Trans Magn 39:2926–2931. doi:10.1109/TMAG.2003.815749
Honkura Y, Mishima C, Hamada N, Gutfleisch O (2005) Texture memory effect of Nd-Fe-B during hydrogen treatment. J Magn Magn Mat 290–291(P2):1282–1285. doi:10.1016/j.jmmm.2004.11.423
Gutfleisch O, Güth K, Woodcock TG, Schultz L (2013) Recycling used Nd-Fe-B sintered magnets via a hydrogen-based route to produce anisotropic, resin bonded magnets. Adv Energy Mater 3:151–155. doi:10.1002/aenm.201200337
Sheridan RS, Sillitoe R, Zakotnik M, Williams AJ (2012) Anisotropic powder from sintered NdFeB magnets by the HDDR processing route. J Magn Magn Mater 324(1):63–67. doi:10.1016/j.jmmm.2011.07.043
Sheridan RS, Williams AJ, Harris IR, Walton A (2014) Improved HDDR processing route for production of anisotropic powder from sintered NdFeB type magnets. J Magn Magn Mater 350:114–118. doi:10.1016/j.jmmm.2013.09.042
Walton A, Han Y, Rowson NA, Speight JD, Mann VSJ, Sheridan RS, Bradshaw A, Harris IR, Williams AJ (2015) The use of hydrogen to separate and recycle neodymium-iron-boron-type magnets from electronic waste. J Clean Prod 104:236–241. doi:10.1016/j.jclepro.2015.05.033
Dirba I, Sawatzki S, Gutfleisch O (2014) Net-shape and Crack-Free production of Nd-Fe-B magnets by hot deformation. J Alloys Compd 589:301–306. doi:10.1016/j.jallcom.2013.11.188
Gutfleisch O, Kirchner A, Grünberger W, Hinz D, Nagel H, Thompson P, Chapman JN, Müller KH, Harris IR, Schultz L (1998) Textured NdFeB HDDR magnets produced by die-upsetting and backward extrusion. J Phys D 31:807–811. doi:10.1088/0022-3727/31/7/009
El-Aziz AM, Kirchner A, Gutfleisch O, Gebert A, Schultz L (2000) Investigations of the corrosion behaviour of nanocrystalline Nd-Fe-B hot pressed magnets. J Alloys Comd 311:299–304. doi:10.1016/S0925-8388(00)01112-9
Constantinides S (2016) Permanent magnets in a changing world market. Magnetics Magazine: Business and Technology (Spring 2016, Feb 26, 2016). http://www.magneticsmagazine.com/main/articles/permanent-magnets-in-a-changing-world-market. Accessed 8 May 2016
Bandara HMD, Darcy JW, Apelian D, Emmert MH (2014) Value analysis of neodymium content in shredder feed: towards enabling the feasibility of rare earth magnet recycling. Environ Sci Technol 48:6553–6560. doi:10.1021/es405104k
Constantinides S (2012) The Demand for Rare Earth Materials in Permanent Magnets. Presentation at the 51st annual conference of metallurgists (COM 2012), Sept. 30–Oct. 3, 2012. Niagara Falls. http://www.arnoldmagnetics.com/Portals/0/Files/Tech%20Library/Technical%20Publications/Tech%20Papers/Demand%20for%20rare%20earth%20materials%20in%20permanent%20magnets%20-%20Constantinides%20-%20COM%20-%202012%20psn%20hi-res.pdf?ver=2015-09-21-101252-140. Accessed 17 Sept 2016
OICA (2016) Sales of new vehicles 2005–2015. http://www.oica.net/category/sales-statistics. Accessed 23 May 2016
Du X, Graedel TE (2011) Global rare earth in-use stocks in NdFeB permanent magnets. J Ind Ecol 15(6):836–843. doi:10.1111/j.1530-9290.2011.00362.x
Guyonnet D, Planchon M, Rollat A, Escalon V, Tuduri J, Charles N, Vaxelaire S, Dubois D, Fargier H (2015) Material flow analysis applied to rare earth elements in Europe. J Clean Prod 107:215–228. doi:10.1016/j.jclepro.2015.04.123
Habib K, Wenzel H (2014) Exploring rare earths supply constraints for the emerging clean energy technologies and the role of recycling. J Clean Prod 84:348–359. doi:10.1016/j.jclepro.2014.04.035
Bandara HMD, Mantell MA, Darcy JW (2015) Closing the lifecycle of rare earth magnets: discovery of neodymium in slag from steel mills. Energy Technol 3:118–120. doi:10.1002/ente.201402162
Habib K, Parajuly K, Wenzel H (2015) Tracking the flow of resources in electronic waste - the case of end-of-life computer hard disk drives. Environ Sci Technol 49(20):12441–12449. doi:10.1021/acs.est.5b02264
Pålsson BI, Wanhainen C, Yurramendi L, Guarde D, Egia A (2015) Cryo-grinding of waste from electronic and electric equipment, implications for recovery of rare earth elements. In: Physical Separation’15, Falmouth, Cornwall, 26 pp
Ueberschaar M, Rotter VS (2015) Enabling the recycling of rare earth elements through product design and trend analyses of hard disk drives. J Mater Cycles Waste Manag 17(2):266–281. doi:10.1007/s10163-014-0347-6
Baba K, Nemoto T, Maruyama H, Taketani N, Itayagoshi K, Hirose Y (2010) Hitachi’s involvement in material resource recycling. Hitachi Rev 59(4): 180–187. www.hitachi.com/rev/pdf/2010/r2010_04_110.pdf. Accessed 8 May 2016
Baba K, Hiroshige Y, Nemoto T (2013) Rare-earth magnet recycling. Hitachi Rev 62(8): 452–455. www.hitachi.com/rev/pdf/2013/r2013_08_105.pdf. Accessed 8 May 2016
Abrahami ST (2012) Rare-earths recovery from post-consumer HDD scrap. Master thesis. Department of Materials Science and Engineering, TU Delft
Abrahami ST, Xiao Y, Yang Y (2015) Rare-earth elements recovery from post-consumer hard-disc drives. Miner Process Extr Metall (Trans. Inst. Min. Metall. C) 124(2):106–115. doi:10.1179/1743285514Y.0000000084
Walton A, Campbell A, Sheridan RS, Mann VSJ, Speight JD, Harris IR, Guerrero B, Bagan C, Conesa A, Schaller V (2014) Recycling of rare earth magnets. In: Proceeding od the 23rd international workshop on rare earth magnets and their applications, Annapolis, pp 26–30
Zakotnik M, Harris IR, Wiliams AJ (2009) Multiple recycling of NdFeB-type sintered magnets. J Alloys Compd 469:314–321. doi:10.1016/j.jallcom.2008.01.114
Walton A, Han Y, Speight JD, Harris IR, Williams AJ (2012) The use of hydrogen to extract and re-process NdFeB magnets from electronic waste. In: Proceeding of the 22nd international Workshop on Rare Earth Permanent Magnets and Their Applications. Nagasaki, pp 11–13
Högberg S, Bendixen FB, Mijatovic N, Jensen BB, Holbøll J (2015) Influence of demagnetization-temperature on magnetic performance of recycled Nd-Fe-B magnets. In: Proceeding of the IEEE international electric machines and drives conference. pp 1242–1246
Meakin JP, Speight JD, Sheridan RS, Bradshaw A, Harris IR, Williams AJ, Walton A (2016) 3-D laser confocal microscopy study of the oxidation of NdFeB magnets in atmospheric conditions. Appl Surf Sci 378:540–544. doi:10.1016/j.apsusc.2016.03.182
Herraiz E, Degri M, Bradshaw A, Sheridan RS, Mann VSJ, Harris IR, Walton A (2016) Recycling of rare earth magnets by hydrogen processing and re-sintering. In: 24th international workshop on rare earth permanent magnet and their applications, Darmstadt
Mottram RS, Kianvash A, Harris IR (1999) The use of metal hydrides in powder blending for the production of NdFeB-type magnets. J Alloys Compd 283:282–288. doi:10.1016/S0925-8388(98)00910-4
Zakotnik M, Tudor CO (2015) Commercial-scale recycling of NdFeB-type magnets with grain boundary modification yields products with ‘designer properties’ that exceed those of starting materials. Waste Manag 44:48–54. doi:10.1016/j.wasman.2015.07.041
Mann VSJ, Škulj I, Walton A (2016) Production of Sintered magnets from recycled hard disk drive voice coil magnets. In: 24th international Workshop on Rare Earth Permanent Magnets and their Applications. Darmstadt
Kim AS, Kim DH, Namkung S, Jang TS, Lee DH, Kwon HW, Hwang DH (2004) HDDR processing of magnet scrap. IEEE Trans Magn 40:2877–2879
Zakotnik M, Williams AJ, Harris IR (2004) Possible methods of recycling NdFeB-type sintered magnets using the HD/degassing or HDDR processes. In: Proceedings of 18th international Workshop on Rare Earth Permanent Magnets & Their Applications
Li X, Yue M, Zakotnik M, Liu W, Zhang D, Zuo T (2015) Regeneration of waste sintered Nd-Fe-B magnets to fabricate anisotropic bonded magnets. J Rare Earths 33:736–739. doi:10.1016/S1002-0721(14)60478-6
Farr M, Sitarz P, Saje B, Sheridan RS, Bradshaw A, Mann VSJ, Harris IR, Walton A (2016) Production of injection moulded NdFeB magnets from recycled hard disk drive voice coil magnets. In: 24th international Workshop on Rare Earth Magnets and their Applications. Darmstadt
Farr M, Sitarz P, Saje B, Sheridan RS, Bradshaw A, Mann VSJ, Harris IR, Walton A (2016) NdFeB magnets from recycled hard disk drive voice coil magnets. 24th international Workshop on Rare Earth Permanent Magnets and their Applications. Darmstadt
Itoh M, Masuda M, Suzuki S, Machida K-I (2004) Recycling of rare earth sintered magnets as isotropic bonded magnets by melt-spinning. J Alloys Compd 374:393–396. doi:10.1016/j.jallcom.2003.11.030
Bounds C (1994) The recycle of sintered magnet swarf. In: Liddell KC et al (eds) Metals and materials waste reduction, recovery and mediation. TMS, Warrendale, PA, pp 173–186
Bandara HMD, Field KD, Emmert MH (2016) Rare earth recovery from end-of-life motors employing green chemistry design principles. Green Chem 18:753–759. doi:10.1039/C5GC01255D
Yoon HS, Kim CJ, Lee JY, Kim SD, Lee JC (2003) Separation of neodymium from NdFeB permanent magnet scrap. J Korean Inst Resour Recycl 12(6):57–63. doi:10.7844/kirr
Ellis TW, Schmidt FA, Jones LL (1994) Methods and opportunities in the recycling of rare earth based materials. In: Liddell KC et al (eds) Metals and materials waste reduction, recovery, and remediation. TMS, Warrendale, PA, pp 199–206
Lee JC, Kim WB, Jeong J, Yoon IJ (1998) Extraction of neodymium from Nd-Fe-B magnet scraps by sulfuric acid. J Korean Inst Met Mater 36(6):967–972
Koyama K, Kitajima A, Tanaka M (2009) Selective leaching of rare-earth elements from an Nd-Fe-B magnet. Kidorui (Rare Earths) 54:36–37
Koyama K, Tanaka M (2011) In: Machida K (ed) The latest technology trend and resource strategy of rare earths. CMC Press, Tokyo, pp 127–131
Itakura T, Sasai R, Itoh H (2006) Resource recovery from Nd-Fe-B sintered magnet by hydrothermal treatment. J Alloys Compd 408–412:1382–1385. doi:10.1016/j.jallcom.2005.04.088
Prakash V, Sun ZHI, Sietsma J, Yang Y (2016) Simultaneous electrochemical recovery of rare earth elements and iron from magnet scrap: a theoretical analysis (Chap. 22). In: De Lima IB, Filho WL (eds) Rare earths industry technological, economic, and environmental implications. Elsevier, Amsterdam, pp 335–346
Voncken JHL (2016) The rare earth elements, an introduction. SpringerNature, Dordrecht
Cotton S (2006) Lanthanide and actinide chemistry. John Wiley & Sons Ltd, West Sussex
Monecke T, Kempe U, Monecke J, Sala M, Wolf D (2002) Tetrad effect in rare earth element distribution patterns: a method of quantification with application to rock and mineral samples from granite-related rare metal deposits. Geochim Cosmochim Acta 66:1185–1196. doi:10.1016/S0016-7037(01)00849-3
Kraikaew J, Srinuttrakul W, Chayavadhanakur C (2005) Solvent extraction study of rare earths from nitrate medium by the mixtures of TBP and D2EHPA in kerosene. J Metals Mater Miner 15(2):89–95
Nash KL, Choppin GR (1995) Separation of f elements. Springer Science + Business Media. (eBook). doi 10.1007/978-1-4899-1406-4
Mohammadi M, Forsberg K, Kloo L, Martinez De La Cruz J, Rasmuson A (2015) Separation of ND(III), DY(III) and Y(III) by solvent extraction using D2EHPA and EHEHPA. Hydrometallurgy 156:215–224. doi:10.1016/j.hydromet.2015.05.004
Gupta CK, Krishnamurthy N (2005) Extractive metallurgy of rare earths. CRC Press, London
Xie F, Zhang TA, Dreisinger D, Doyle F (2014) A critical review on solvent extraction of rare earths from aqueous solutions. Miner Eng 56:10–28. doi:10.1016/j.mineng.2013.10.021
Yoon HS, Kim CJ, Chung KW, Kim SD, Lee JY, Kumar JR (2016) Solvent extraction, separation and recovery of dysprosium (Dy) and neodymium (Nd) from aqueous solutions: waste recycling strategies for permanent magnet processing. Hydrometallurgy. doi:10.1016/j.hydromet.2016.01.028
Banda R, Jeon H, Lee M (2012) Solvent extraction separation of Pr and Nd from chloride solution containing La using Cyanex 272 and its mixture with other extractants. Sep Purif Technol 98:481–487. doi:10.1016/j.seppur.2012.08.015
Liu Y, Jeon HS, Lee MS (2014) Solvent extraction of Pr and Nd from chloride solution by the mixtures of Cyanex 272 and amine extractants. Hydrometallurgy 150:61–67. doi:10.1016/j.hydromet.2014.09.015
Abreu RD, Morais CA (2014) Study on separation of heavy rare earth elements by solvent extraction with organophosphorous acids and amine reagents. Miner Eng 61:82–87. doi:10.1016/j.mineng.2014.03.015
Elwert T, Goldmann D, Römer F (2014) Separation of lanthanides from NdFeB magnets on a mixer-settler plant with PC-88A. World Metall 67:287–296
Zhu ZX, Sasaki Y, Suzuki H, Suzuki S, Kimura T (2004) Cumulative study on solvent extraction of elements by N, N, N’, N’-tetraoctyl-3-oxapentanediamide (TODGA) from nitric acid into n-dodecane. Anal Chim Acta 527:163–168. doi:10.1016/j.aca.2004.09.023
Dam HH, Reinhoudt DN, Verboom W (2007) Multicoordinate ligands for actinide/lanthanide separations. Chem Soc Rev 36:367–377. doi:10.1039/B603847F
Modolo G, Vijgen H, Serrano-Purroy D, Christiansen B, Malmbeck R, Sorel C, Baron P (2007) DIAMEX counter-current extraction process for recovery of trivalent actinides from simulated high active concentrate. Sep Sci Technol 42:439–452. doi:10.1080/01496390601120763
Sinkov SI, Rapko BM, Lumetta GJ, Hay BP, Hutchison JE, Parks BW (2004) Bicyclic and acyclic diamides: comparison of their aqueous phase binding constants with Nd(III), Am(III), Pu(IV), Np(V), Pu(VI), and U(VI). Inorg Chem 43:8404–8413. doi:10.1021/ic049377m
Tyumentsev MS, Foreman MRS, Ekberg C, Matyskin AV, Retegan T, Steenari B-M (2016) The solvent extraction of rare earth elements from nitrate media with novel polyamides containing malonamide groups. Hydrometallurgy 164:24–30. doi:10.1016/j.hydromet.2016.05.007
Kim D, Powell LE, Delmau LH, Peterson ES, Herchenroeder J, Bhave RR (2015) Selective extraction of rare earth elements from permanent magnet scraps with membrane solvent extraction. Environ Sci Technol 49(16):9452–9459. doi:10.1021/acs.est.5b01306
Nakashima K, Kubota F, Maruyama T, Goto M (2003) Ionic liquids as a novel solvent for lanthanide extraction. Anal Sci 19(8):1097–1098. doi:10.2116/analsci.19.1097
Kubota F, Baba Y, Goto M (2012) Application of ionic liquids for the separation of rare earth metals. Solvent Extr Res Dev, Jpn 19:17–28. doi:10.1252/jcej.11we005
Baba Y, Kubota F, Kamiya N et al (2011) Recent advances in extraction and separation of rare-earth metals using ionic liquids. J Chem Eng Jpn 44(10):679–685. doi:10.1252/jcej.10we279
Park J, Jung Y, Kusumah P, Lee J, Kwon K, Lee CK (2014) Application of ionic liquids in hydrometallurgy. Int J Mol Sci 15:15320–15343. doi:10.3390/ijms150915320
Makanyire T, Sanchez-Segado S, Jha A (2016) Separation and recovery of critical metal ions using ionic liquids. Adv Manuf 4(1):33–46. doi:10.1007/s40436-015-0132-3
Yoon SJ, Lee JG, Tajima H, Yamasaki A, Kiyono F, Nakazato T, Tao H (2010) Extraction of lanthanide ions from aqueous solution by bis(2-ethylhexyl)phosphoric acid with room-temperature ionic liquids. J Ind Eng Chem 16(3):350–354. doi:10.1016/j.jiec.2009.09.063
Sasaki Y, Sugo Y, Suzuki S, Tachimori S (2001) The novel extractants, diglycolamides, for the extraction of lanthanides and actinides in HNO3–n-dodecane system. Solvent Extr Ion Exch 19:91–103. doi:10.1081/SEI-100001376
Naganawa H, Shimojo K, Mitamura H, Sugo Y, Noro J, Goto M (2007) A New, “green” extractant of the diglycol amic acid type for lanthanides. Solvent Extr Res Dev, Jpn 14:151–159
Shimojo K, Naganawa H, Noro J, Kubota F, Goto M (2007) Extraction behavior and separation of lanthanides with a diglycol amic acid derivative and a nitrogen-donor ligand. Anal Sci 23(12):1427–1430. doi:10.2116/analsci.23.1427
Kikuchi Y, Matsumiya M, Kawakami S (2014) Extraction of rare earth ions from Nd-Fe-B magnet wastes with TBP in tricaprylmethylammonium nitrate. Solvent Extr Res Dev, Jpn 21:137–145. doi:10.15261/serdj.21.137
Vander Hoogerstraete T, Wellens S, Verachtert K, Binnemans K (2013) Removal of transition metals from rare earths by solvent extraction with an undiluted phosphonium ionic liquid: separations relevant to rare-earth magnet recycling. Green Chem 15:919–927. doi:10.1039/C3GC40198G
Vander Hoogerstraete T, Binnemans K (2014) Highly efficient separation of rare earths from nickel and cobalt by solvent extraction with the ionic liquid trihexyl(tetradecyl)phosphonium nitrate: process relevant to the recycling of rare earths from permanent magnets and nickel metal hydride batteries. Green Chem 16:1594–1606. doi:10.1039/C3GC41577E
Vander Hoogerstraete T, Blanpain B, Van Gerven T, Binnemans K (2014) From NdFeB magnets towards the rare-earth oxides: a recycling process consuming only oxalic acid. RSC Adv 4:64099–64111. doi:10.1039/C4RA13787F
Riano S, Binnemans K (2015) Extraction and separation of neodymium and dysprosium from used NdFeB magnets: an application of ionic liquids in solvent extraction towards the recycling of magnets. Green Chem 17:2931–2942. doi:10.1039/C5GC00230C
Dupont D, Binnemans K (2015) Recycling of rare earths from NdFeB magnets using a combined leaching/extraction system based on the acidity and thermomorphism of the ionic liquid [Hbet][Tf2 N]. Green Chem 17:2150–2163. doi:10.1039/C5GC00155B
Okabe TH and Shirayama S (2011) Method and apparatus for recovery of rare earth element, US Patent Application Publication: US2011/0023660A1
Murase K, Machida K, Adachi G (1995) Recovery of rare metals from scrap of rare earth intermetallic material by chemical vapour transport. J Alloys Compd 217:218–225. doi:10.1016/0925-8388(94)01316-A
Önal MAR, Borra CR, Guo M, Blanpain B, Van Gerven T (2015) Recycling of NdFeB magnets using sulfation, selective roasting, and water leaching. J Sustain Metall 1:199–215. doi:10.1007/s40831-015-0021-9
Ellis TW, Schmidt FA (1995) Recycling of rare earth metals from rare earth-transition metal alloy scrap by liquid metal extraction. U.S. Patent 5,437,709
Xu Y, Chumbley LS, Laabs FC (2000) Liquid metal extraction of Nd from NdFeB magnet scrap. J Mater Res 15:2296–2304. doi:10.1557/JMR.2000.0330
Takeda O, Okabe TH, Umetsu Y (2006) Recovery of neodymium from a mixture of magnet scrap and other scrap. J Alloys Compd 408–412:387–390. doi:10.1016/j.jallcom.2005.04.094
Takeda O, Okabe TH, Umetsu Y (2004) Phase equilibrium of the system Ag-Fe-Nd, and Nd extraction from magnet scraps using molten silver. J Alloys Compd 379:305–313. doi:10.1016/j.jallcom.2004.02.038
Moore M, Gebert A, Stoica M et al (2015) A route for recycling Nd from Nd-Fe-B magnets using Cu melts. J Alloys Compd 647:997–1006. doi:10.1016/j.jallcom.2015.05.238
Hua Z, Wang J, Wang L, Zhao Z, Li X, Xiao Y, Yang Y (2014) Selective extraction of rare earth elements from NdFeB scrap by molten chlorides. ACS Sustain Chem Eng 2:2536–2543. doi:10.1021/sc5004456
Tanaka M, Sato Y, Huang Y, Narita H, Koyama K (2009) In: Proceedings of the 10th international symposium on east asian resources recycling technology (EARTH2009). The Korean Institute of Resources Recycling, Seoul, pp 200–203
Hua ZS, Wang L, Wang J, Xiao YP, Yang YX, Zhao Z, Liu MJ (2015) Extraction of rare earth elements from NdFeB scrap by AlF3–NaF melts. Mater Sci Technol 31:1007–1010. doi:10.1179/1743284714Y.0000000672
Saito T, Sato H, Motegi T (2006) Recovery of rare earths from sludges containing rare-earth elements. J Alloys Compd 425:145–147. doi:10.1016/j.jallcom.2006.01.011
Yang Y, Abrahami ST, Xiao Y (2013) Recovery of rare earth elements from EOL permanent magnets with molten slag extraction. In: Proceedings of the 3rd international slag valorisation symposium. Leuven, pp 249–252. http://www.slag-valorisation-symposium.eu/2013/images/papers/ps3_1_Yang.pdf. Accessed 8 May 2016
Bian Y, Guo S, Jiang L, Liu J, Tang K, Ding W (2016) Recovery of rare earth elements from NdFeB magnet by VIM–HMS method. ACS Sustain Chem Eng 4:810–818. doi:10.1021/acssuschemeng.5b00852
Jakobsson LK, Kennedy MW, Aune RE, Tranell G (2016) Recovery of rare earth elements from the ferrous fraction of electronic waste. In: Kirchain RE et al (eds) REWAS 2016-towards materials resource sustainability. TMS, Warrendale, PA, pp 89–93
Kobayashi S, Kobayashi K, Nohira T, Hagiwara R, Oishi T, Konishi H (2011) Electrochemical formation of Nd-Ni Alloys in molten LiF-CaF2-NdF3. J Electrochem Soc 158:E142–E146. doi:10.1149/2.072112jes
Finnveden G, Hauschild MZ, Ekvall T, Guinee J, Heijungs R, Hellweg S, Koehler A, Pennington D, Suh S (2009) Recent developments in life cycle assessment. J Environ Manag 91:1–21. doi:10.1016/j.jenvman.2009.06.018
Bast U (2014) Recycling von Komponenten und strategischen Metallen aus elektrischen Fahrantrieben: MORE (Motor Recycling). Final Research Report. http://edok01.tib.uni-hannover.de/edoks/e01fb15/826920594.pdf. Accessed 8 May 2016
Walachowicz F, March A, Fiedler S, Buchert M, Sutte J, Merz C (2014) Recycling von Elektromotoren-MORE: Ökobilanz der Recyclingverfahren. Final Report, Berlin. http://de.slideshare.net/AndrewMarch/morelcaendberichtfinal17okt2014. Accessed 8 May 2016
Sprecher B, Xiao Y, Walton A, Speight J, Harris R, Kleijn R, Visser G, Kramer GJ (2014) Life Cycle inventory of the production of rare earths and the subsequent production of NdFeB rare earth permanent magnets. Environ Sci Technol 48:3951–3958. doi:10.1021/es404596q
Acknowledgments
The efforts leading to this publication have received funding from the European Community’s Seventh Framework Programme ([FP7/2007–2013]) under Grant Agreement No. 607411 (MC-ITN EREAN: European Rare Earth Magnet Recycling Network) (project website of EREAN: http://www.erean.eu ). The first author (Yongxiang Yang) also acknowledges the EU FP7 project REEcover (Project No. 603564) for providing the financial support during the preparation of this paper (project website of REEcover: http://www.reecover.eu ). This publication reflects only the authors’ views, exempting the Community from any liability. Discussions with Mr. Steve Constantinides from Arnold Magnetics Technologies are appreciated regarding global market demands and development for NdFeB permanent magnets.
Author information
Authors and Affiliations
Corresponding author
Additional information
The contributing editor for this article was Bernd Friedrich.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Yang, Y., Walton, A., Sheridan, R. et al. REE Recovery from End-of-Life NdFeB Permanent Magnet Scrap: A Critical Review. J. Sustain. Metall. 3, 122–149 (2017). https://doi.org/10.1007/s40831-016-0090-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-016-0090-4