Abstract
Fabrication of a new nanocomposite architecture comprising small weight percentage (< 0.5 wt%) of Toluidine Blue O dye as a filler in host polymer poly(vinyl alcohol) (PVA) via a simple route based on solution-casting is presented. Their microstructure, electrical, linear optical and third-order nonlinear optical properties are introduced here. These samples were characterized as nanocomposites, with dye chromophore uniformly distributed in the molecular chains of the polymer PVA. The effect of fluorescence quenching due to H-aggregates is also discussed. Low threshold third order nonlinear optical response of these nanocomposite film samples was carried out employing the Z-scan technique. With increase in intensity, a transition from reverse saturable absorption to saturable absorption was observed in the nanocomposite films, on excitation with continuous wave (CW) He–Ne laser light. The nonlinear refraction based optical limiting of CW laser light is demonstrated using aperture limited geometry. Compared with other reported results, the estimated values of the nonlinear refractive index (n2), nonlinear absorption coefficients (β), third order nonlinear susceptibility (χ(3)) and limiting threshold are much better. These results thus possess tremendous scope towards future fabrication of optoelectronic and photonic devices such as organic/polymer light-emitting devices, dye-sensitized solar cells, optical limiters, optical switches, etc.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Several studies on organic nanomaterials have been widely reported owing to their application as building blocks for different kinds of integrated photonic and optoelectronic devices mainly as a light waveguide, microscale light source, optical routers, photonic transistors, logical gates, multiplexers, photonic sensors and photoelectronic converters [1, 2]. Over the past decades, polymer nanocomposites have drawn notable progress in scientific and industrial communities regarding optoelectronic and photonic technology, owing to their remarkable improvements on numerous functionalities such as optical, electrical and so on, without sacrificing the advantages of the polymer as well as inorganic/organic moieties [3, 4]. The use of very low filler loadings in the host polymer matrix often leads to enhancement in the photophysical properties, as well as overall photostability of the filler molecules, which when coupled with the ability to cast these composites into various forms like films, have made it possible to utilize them in a wide range of applications. The studies on emission profile of organic dye-polymer nanocomposite architecture plays an important role in knowing the molecular aggregation [5]. Fluorescence quenching in organic dyes is essential to understand as this process can be used to detect molecular recognition in sensors, investigation of accessibility and localization of probes in a membrane or protein, etc. [6]. An understanding of the mechanism behind the enhancement and the quenching of emission is necessary to obtain an insight into the interaction between light and fluorophore (organic dye) nanocomposites for applications in the field of optoelectronic and photonic devices. Besides this, addition of filler molecules in polymer can be utilized to promote morphology refinement with no adverse effect in the polymer structure. In organic-polymer interface, the amorphous nature increases the flexibility of the polymer backbone and promotes the transfer of ions [7, 8].
Incorporating organic molecules in host polymer and developing their nanocomposite architecture provide a new avenue towards the design of nonlinear optical (NLO) materials, due to their biocompatibility, architectural flexibility, facile mode of processing, and diverse functionality [9, 10]. The excellent NLO properties of organic dye-polymer nanocomposites marked them as potential candidates for optoelectronic and photonic device applications involving low intensity light sources, optical limiters, optical switches, organic light-emitting diodes (OLEDs), electroluminescent devices, photovoltaic, solar cells, polymer-based nanodevices, and in display technologies, recording and storage of information, etc. [11]. Poly(vinyl alcohol) (PVA) was preferred as a host material/solvent for this work, since this polymer possess unique physical properties including high optical transparency, chemical resistance, interesting mechanical property, flexible, biodegradable, biocompatible, good gas barrier property against ambient gases, high dielectric strength, dopant dependent electrical and optical properties and excellent film forming property, which arises from the presence of OH groups and the hydrogen bond formation [11, 12]. Among several methods, solution-casting is recognized as one of the easy, facile and cost-effective route to prepare the polymer nanocomposite films [12,13,14].
The materials (guest as well as host) chosen for preparing the new combination presented in this article are most familiar materials in medical fields [15,16,17,18]. Toluidine Blue O (TBO) dye (C15H16N3SCl) also named as 3-Amino-7(dimethyl amino)-2-methyl phenothiazin-5-ium chloride, a dark-blue coloured positively charged basic cationic thiazine dye of the quinone-imine family was selected (as solute) since they absorb in the higher wavelength regime in visible spectrum. This dye is extensively used in the medical research/practice especially as stain [16,17,18]. In this article, we developed a new combination of PVA based organic dye nanocomposite solid films, and their surface morphology, electronic and optical properties were investigated. We have already reported the nonlinear refraction behaviour of TBO-PVA nanocomposite films using a 442 nm He-Cd CW laser light excitation [19]. A considerable amount of work has been performed on the structure and linear optical properties of TBO dye by many researchers, but there are only few reports devoted to the study of third-order NLO properties of TBO dye as of now [16, 20, 21]. Our findings reported here are expected to be the first effort done for studying low threshold nonlinear absorption properties of the solid films of TBO-PVA nanocomposite systems, to the best of our knowledge. Even though our previous report gives an idea on third order NLO properties of this combination of TBO dye-PVA nanocomposite system [19], here in this article the study on structure, morphology, electrical and optical properties (linear as well nonlinear) are discussed in detail. The excitation wavelength for NLO studies of these nanocomposite samples is in the near resonance region and the effect of H-aggregates is also mentioned in this paper. The report has significant impact on third-order nonlinear absorption behaviour as these dye-PVA samples show a transition from RSA to SA, which is rarely seen under low power CW laser light excitation [22,23,24]. The optical limiting action achieved by nonlinear refraction behaviour is also demonstrated at the end.
1.1 Experimental details
A known amount (5 wt%) of PVA (molecular weight = 125,000 g/mol s.d. Fine chemicals, Mumbai, India) was dissolved into the desired quantity of distilled water at 75 °C. The hot solution was stirred under magnetic stirring for 2 h, until the polymer is completely dissolved and forms a clear viscous solution; followed by cooling to room temperature with continuation of stirring for another 1 h. By incorporating very small wt% (< 0.5 wt%) of Toluidine Blue O (TBO) dye (Sigma Aldrich, USA) in the host polymer PVA solution, the nanocomposite films of TBO-PVA systems for the present work were fabricated via a simple and cost-effective method based on solution-casting. This dye-polymer mixture was ultrasonically agitated and then deposited onto 7.5 × 2.5 cm2 perfectly leveled micro-slide glass substrates in a dark, dust free environment. Thus we gained good quality transparent films of uniform surface finish, with thickness in the range ~ 90 μm for about five to six days. However, this casting method was improved to allow film formation for studying NLO properties [19]. A reaction scheme illustrating possible weak hydrogen bonding interactions of TBO dye chromophores with PVA to form complexes is sketched in Fig. 1 (created using ChemSketch). Similar weak bonding interactions are reported for PVA based nanocomposite films [13, 25].
The X-ray patterns of the samples were obtained using a PANalytical X’Pert PRO Diffractometer with CuKα radiation of wavelength 1.5406 Å. The scan was taken in the 2θ range 10–80° with a scanning step size of 0.017°. The samples were analyzed using the HRTEM (JOEL, JEM-2100) technique, to get detailed information regarding the surface and homogeneity of the nanocomposite films. The linear absorption and photoluminescence (PL) emission spectra of the samples were acquired using a SHIMADZU UV-2401 PC spectrophotometer and Horiba scientific Fluoromax 4 spectrofluorometer, respectively. The fundamental optical constants required for the analysis of the Z-scan data of the samples were obtained from the optical absorption measurements. The electrical conductivity of the unfilled PVA film as well as TBO-PVA nanocomposite films were measured using a Keithley model 617 electrometer. Low threshold third order nonlinear optical properties of TBO–PVA nanocomposite films were investigated using the standard Z-scan technique. The method involves sending a focused Gaussian laser beam through the sample and measuring the transmittance of the sample with and without an aperture placed in the far field (closed and open aperture schemes, respectively) as a function of the sample position Z, determined with respect to the focal plane of the laser beam. The Z-scan experiments were performed using the Gaussian beam from a He–Ne CW laser light operating at 632.8 nm wavelength and producing a maximum output power of 22 mW. This input beam was focused onto a spot size of 28.79 μm with a biconvex lens of focal length 5 cm yielding a Rayleigh range (Z0) of 4.11 mm, which was greater than the sample thickness, in accordance with the thin sample approximation condition required for the Z-scan theory [11, 12, 26]. Optical limiting based on nonlinear refraction is demonstrated with an aperture limited geometry.
2 Results and discussion
The XRD patterns of unfilled PVA, TBO-PVA nanocomposite films and that of TBO dye powder are presented in Fig. 2. The observed XRD pattern of unfilled PVA film (see Fig. 2a) displays a relatively sharp and broad peak centered at 2θ ~ 19.5° (corresponding to 101 spacing), characteristic of its semicrystalline nature [13]. The several sharp peaks observed in the XRD pattern of the TBO dye in powder form shows that it is polycrystalline in nature (see Fig. 2g). However, when incorporated in the polymer matrix, the crystalline peaks characteristic of the dye molecules disappears (see Fig. 2b–f) [25, 27]. It is evident from figure that more amorphous phase exists in the nanocomposite films, which may be due to the formation of a flexible network in the polymer matrix (or weak hydrogen bond formation). According to the literature, owing to free-volume existing at interface between polymer chain and flexible local PVA chain segmental motion in the nanocomposite polymer electrolyte, the characteristic of the polymer nanocomposite films shows excellent ionic conductivity property [28]. Similar kind of reduction in crystallinity of polymer composites (prepared using solution-casting technique) was reported for other nanocomposites [27, 28]. To determine the size of the filler content in polymer blend, the Debye–Scherrer formula was used [29]. Even though small percent of filler content is added to the host polymer PVA, it can affect the crystallization kinetics of polymer matrix [30]. The crystalline size of TBO dye in powder form and that of TBO-PVA nanocomposite film for 6.539 × 10−5 M TBO dye concentration are evaluated to be ~ 32.97 nm and ~ 11.87 nm, respectively. The effect of concentration of TBO dye in PVA matrix was studied and found that the size is increased up to 25.725 nm (see Table 1).
The photographs of TBO-PVA nanocomposite solution taken before casting solid films and that of good quality transparent TBO–PVA nanocomposite films with uniform surface finish, casted onto 7.5 × 2.5 cm2 glass substrate are shown in Fig. 3i–ii. The HRTEM micrograph of TBO-PVA nanocomposites in solution form at 200 nm magnification (Fig. 3iii) and that in film form at both 200 nm and 20 nm magnifications (see Fig. 3iv) for 1.620 × 10−4 M TBO dye content illustrate a uniform distribution of TBO dye chromophores in the PVA matrix. It was observed that TBO dye chromophores are wrapped by the PVA and distributed uniformly in the polymer matrix (see Fig. 3iv). The particle size measured as < 20 nm was obtained from the HRTEM, and is in good agreement with XRD analyses. Due to the weak interaction between the dye molecules and host PVA, the organic dye chromophores get trapped in the free space/voids of host polymer PVA and hence the porosity is reduced. On increasing the concentration of dye content in host polymer, more dye chromophores may occupy in the interstitial sites of polymer forming higher aggregates [31]. Moreover, encapsulation of dye chromophores in the host polymer improved the structure as well as physical (electrical, optical, mechanical, etc.) properties of the nanocomposite films, in particular, improved the photo-stability and resistance against environmental degradation, compared to its constituent materials [13, 14]. Similar TEM pattern was reported for other material and polymer nanocomposite films [1, 32].
Photographs of (i) TBO-PVA nanocomposite solutions (before casting solid films) and (ii) TBO-PVA nanocomposite films for three different concentrations of TBO dye content: (a) 1.620 × 10−4 M, (b) 3.140 × 10−4 M and (c) 2.125 × 10−3 M. HRTEM micrographs of TBO-PVA nanocomposite for 1.620 × 10−4 M TBO dye content at different magnification ((iii) in solution form and those of (iv) solid film)
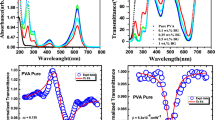
Figure 4 illustrates the linear absorption spectra of TBO-PVA nanocomposite films for different concentrations of TBO dye content along with that of unfilled PVA film and TBO dye in aqueous solution. The well-defined curves of the absorption spectra of TBO-PVA nanocomposite films indicate uniform distribution and homogeneity of TBO dye content in the host polymer PVA. The red line represents the wavelength range of He–Ne CW laser light used for studying NLO properties. The band edge around 295.8 nm (indicating the presence of PVA in Fig. 4a) shifts towards higher wavelength (i.e., 300 nm) as the concentration of TBO dye was increased. This shift may be ascribed to the formation of inter/intramolecular hydrogen bonding between the TBO dye molecule and the OH groups in the host polymer PVA (see Fig. 4b–d) [14, 33, 34]. In Fig. 4e, the TBO dye in aqueous solution possesses three absorption bands centered at ~ 245 nm, 287 nm and 634 nm. The absorption maxima of TBO dye in the nanocomposite films which occur in the orange region at 628 nm, shows a blue shift/hypsochromic shift of ~ 6 nm, in comparison with the aqueous solution of the dye. As there is no peak shift, it is clear from the Fig. 4f that the nanocomposite films have prolonged durability even after 5 years or more. Meanwhile, it may be noted that a shoulder appearing at a lower wavelength develops gradually into an absorption band centered at 592 nm, which indicates the presence of H-aggregates (face-on orientation/‘side-by-side’ anti-parallel arrangement) [34, 35]. It is worth mentioning that a similar interesting behaviour was previously reported by our group for Amido Black 10B dye-PVA films [11].
Linear absorption spectra of (a) unfilled PVA film, TBO-PVA nanocomposite films for different concentrations of TBO dye content (b) 1.620 × 10−4 M, (c) 3.140 × 10−4 M, (d) 2.125 × 10−3 M and those of TBO dye in aqueous solution (e) 6.250 × 10−5 M and (f) TBO-PVA nanocomposite film having 2.125 × 10−3 M TBO dye content taken after 5 years
Figures 5 and 6 show the PL emission spectra of unfilled PVA film and that of TBO-PVA nanocomposite films for different concentrations of TBO dye content, when excited at 300 nm and 560 nm, respectively. The nanocomposite samples displayed violet emission (~ 368 nm and 396 nm), when excited near the absorption maximum (λmax = 300 nm) (see Fig. 5). These films also exhibit strong PL emission in the orange-red region (centered at ~ 647 nm), when excited near its absorption maximum (λmax = 560 nm) (see Fig. 6). From the literature, the mirror image may imply that the structure change between the ground state and excited singlet state is small [35]. However, the host matrix PVA (unfilled) film does not show any emission in the higher wavelength range. As the concentration of the dye content in the nanocomposite films increases, the spectra reveal luminescence quenching, with a progressive change in the emission maxima. It may be noted that, at relatively higher dye contents, the polymer chain cannot effectively prevent dye aggregation, and this may result in the fluorescence quenching at higher concentrations (see Figs. 5f, 6f). According to literature, the non-fluorescent H-aggregates are mainly responsible for the monomer fluorescence quenching in organic dyes [11, 36]. Organic dye-polymer nanocomposite films that emits in the visible region of the spectrum are effectively considered as an excellent medium, as they can provide stable sources of light for displays and illumination sources at a significantly lower cost [13]. The TBO dye exhibits PL emission in the violet region at 397 nm and also in the red region at 680 nm, when excited at 300 nm and 560 nm, respectively (see Figs. 5g, 6g).
In addition, to characterize the semiconducting nature of the films, the bandgap energy (Eg) can be evaluated using the method of Tauc’s plot [33]. The value of Eg for unfilled PVA film and that of TBO dye are found to be 4.04 eV and 1.81 eV, respectively. The TBO–PVA nanocomposite films also exhibited bandgap energy in the range of 2.89–2.65 eV (see Table 1). From the literature, the decrease in band gap energy on increasing concentrations of filler dye content in the nanocomposite films can be attributed to the inter/intramolecular hydrogen bonding between the dye molecules and the polymer [37]. Furthermore, as the number of free ions in polymer matrix increases, the electrical conductivity of TBO–PVA nanocomposite films increases from 1.13 × 10−4 Sm−1 to 1.06 × 10−3 Sm−1, which matches with that of the semiconductors (see Table 1). In other words, addition of filler moieties reduces the intermolecular interaction between the PVA molecules themselves or increases the volume required for ionic carriers to drift in the polymer matrix. This enhances the ionic mobility resulting increase in conductivity [13, 38, 39]. The conductivity of these nanocomposite films is much better than those reported for many organic nanocomposite films [39,40,41].
The open aperture (OA) Z-scan profiles of TBO-PVA nanocomposite films for three different concentrations of TBO dye content: 1.620 × 10−4 M, 3.140 × 10−4 M and 2.125 × 10−3 M, at three different peak incident intensities, I0 = 0.4316 KW/cm2, I0 = 0.8139 KW/cm2 and I0 = 1.1365 KW/cm2 are displayed in Fig. 7. Using Eq. (1), the solid curves are drawn to show the best theoretical fit to the experimental data. As the sample approaches the focus, the transmission decreases forming a well-defined normalized valley, indicating the beginning of an enhanced absorption process like RSA; however, at closer distances from the focus, there is a flip-flop and a sudden enhancement in transmission occurs, depicting the onset of a SA type of nonlinear absorption in which the transmission increases with incident intensity at and around Z0 (see Fig. 7b–d). For the lowest molar concentration (1.620 × 10−4 M), the OA Z-scan profiles were exactly same depicting a cross-over from RSA to SA, for all different I0, studied. However, in Fig. 7d, corresponding to 3.140 × 10−4 M TBO dye concentration, as the on-axis incident peak incident intensity I0, is increased to 1.1365 KW/cm2, interestingly the RSA observed little away from Z0, disappears; instead, a dip corresponding decreased transmission (RSA) appears at Z0. On the other hand, for the highest molar concentration of 2.125 × 10−3 M studied, SA occurs for the lowest I0; and a dip at Z0 is formed with increase of I0, which disappears again for the highest I0 studied (see Fig. 7d). This flip-flop can be explained on the basis of triplet state excitation in detailed using a four-level model (see Fig. 8) [42, 43]. The unfilled PVA film yielded no Z-scan signals (see Fig. 7a).
Schematic diagram of four-level energy model explaining the T–T triplet transition for the phenothiazine dye [42]
It is worth to note that the PL emission exhibits peak in the range from 630 to 680 nm. Besides, the linear optical properties constitute the study of NLO properties of the nanocomposite systems. Normally, strong pumping led to SA rather than RSA, as the excitation wavelength is in the near resonance region, where the absorption coefficient is comparatively larger [14]. Herein, the formation of the H-aggregate in these nanocomposite films clearly signaled by the appearance of the additional peak at 592 nm (see Fig. 4), results in the transition from RSA to SA. Once SA saturates, absorption may occur at 592 nm corresponding to the H-band, which is prominent at the highest concentration (2.125 × 10−3 M) (see Fig. 7b). Because of non-fluorescent H-aggregates, the molecules may undergo non-radiative decay through intersystem crossing (ISC) (quenching to triplet T1) after which absorption from T1 to a higher triplet state can occur (schematically shown in Fig. 8), causing the observed RSA under favourable conditions, for intensity 0.8139 KW/cm2 (Fig. 7c). On further increasing intensity (see Fig. 7d), saturation of RSA occurs giving rise to a complete SA behaviour in the end [22]. The evolution of this intense SA is a drawback for the use of this material in optical limiting devices; however, the contemporary presence of RSA and SA at different excitation intensities could find applications in some kind of switching devices [44]. However, the peculiar RSA-SA behavior, which may be attributed to the competition between different effects that occurs for wavelength at Q-band region, has already been reported in literature for pulsed laser light excitation [23, 44,45,46]. Worthwhile, our group has reported a transition from SA to RSA under the aggregate conditions for Amido Black 10B dye-PVA nanocomposite films [11].
Under CW laser light excitation, the thermal effect will increase the excited state absorption (ESA), resulting RSA type behaviour [24, 47, 48]. Hence, the RSA type of nonlinearity observed here in semiconducting TBO-PVA nanocomposite films at 632.8 nm excitation may primarily be attributed to an ESA process actuated by the population build up in the triplet state [11]. However, the SA type of nonlinearity observed at high intensity (around focus), which occurs after the observation of RSA, may be assigned to the saturation of RSA, as has been reported earlier [22, 42]. Herein, the fall of β with I0 may be the consequence of an ESA assisted RSA process. The nonlinear absorption coefficient (β) obtained from the theoretical fit is to be of the order of 10−5 cm/W and also found to be increasing on increasing intensity (see Table 2). It may be noted that the measured values of Is are low (as expected for CW laser light excitation) in the order of 103 W/cm2, and decreases quickly with increasing TBO dye content, same as that reported for Amido Black 10B dye-PVA films [11]. Thus, these TBO-PVA nanocomposite film samples undergo a transformation from RSA to SA performance under aggregation condition. As the superposition of positive and negative nonlinear absorption occurs, the total absorption coefficient can be formulated as [12, 49]:
where the first term denotes negative nonlinear absorption (SA) and the second, positive nonlinear absorption (RSA). Also, α0 is the linear absorption coefficient, I the laser radiation intensity and IS, the saturation intensity, β is the intensity dependent positive nonlinear absorption coefficient.
Figure 9 illustrates the closed aperture (CA) Z-scan profiles of the TBO-PVA nanocomposite films with 2.125 × 10−3 M concentration of TBO dye content at different peak intensities 1.1365 KW/cm2 and 0.3924 KW/cm2. They exhibits peak-valley characteristics, indicating self- defocusing effect (negative nonlinear refraction, n2 < 0). The solid line represents the theoretical fit to the experimental data, with ΔФ0 and Δψ0 as a free fitting parameter, using the expression [53],
The measured values of effective nonlinear refraction coefficient n2 are of the order of 10−6 cm2/W, which are higher than those reported (see Table 2). The real and imaginary parts of χ(3) and the absolute values of χ(3) are evaluated using the relations [54]:
where ε0 is the permittivity in free space, c is the speed of light, n0 is the linear refractive index and λ, the wavelength of light. The nonlinear refraction behaviour can be due to several physical mechanisms such as Kerr effect, thermal self-focusing, defocusing, etc. Here, the peak-valley separation is obtained as ~ 1.75Z0, which is closer to that for an excited state refractive nonlinearity [55, 56]. On excitation with CW laser light, the samples exhibit an asymmetric nature in the CA Z-scan profiles which indicates the dominance of a thermally induced nonlinearity (thermo-optic effect), as reported earlier [56, 57]. The nonlinear absorption coefficient (β), nonlinear refractive index (n2), saturation intensity (Is) and third order nonlinear susceptibility (χ(3)), which are calculated and listed in the Table 2, should be considered as effective values and are comparable to or even better than the recently reported values (see Table 2).
As reported in literature, the optical limiting (OL) (achieved by several factors such as nonlinear refraction, RSA, thermal looming, etc.) for CW laser light plays a vital role for the protection of sensors and even human eye [11, 12, 56]. The optical limiting action of TBO-PVA nanocomposite films with TBO dye concentrations 2.125 × 10−3 M and 3.14 × 10−4 M is presented in Fig. 10. Here, as the samples displayed SA type of behaviour which could not yield optical limiting, the observed OL effect can be ascribed to thermally induced nonlinear refraction [56, 58, 59]. The samples with same concentrations as those used for the Z-scan measurement are employed for the OL study also. From the threshold intensity for optical limiting of each sample, it can be seen that the optical power limiting threshold is inversely proportional to the concentration of the dye content (see Table 3). Moreover, a better limiting threshold has been achieved for higher concentration of TBO dye. The results are tabulated in Table 3 and these values are comparable to or even better than those reported in literature [58, 60, 61]. These observations indicate that TBO-PVA nanocomposite films can be an efficient optical limiter at this wavelength. These results, together with the simple, facile, controllable and flexible approach, signify the scope for utilizing this cost-effective, PVA-based multifunctional nanocomposite with free of surface irregularities, prolonged life-span; as a good platform for applications in optoelectronic and photonic devices including organic/polymer light-emitting devices, optical switching devices, optical sensors, etc.
3 Conclusion
An overview on the fabrication and characterization of a new, low threshold NLO material, comprising TBO dye impregnated in the vinyl hydroxy polymer PVA, is reported here. Compared to the other articles reported on NLO properties of TBO dye-polymer system, the significance of the present samples are their low threshold NLO response (RSA → SA) and emission features near the resonant wavelength. HRTEM and XRD analyses of the nanocomposite films indicate a particle size of < 20 nm. The samples are characterized as spherical shaped nanosized TBO dye chromophores, dispersed uniformly in the free volumes/interstitial sites available between the larger molecules (molecular chains) of the host polymer PVA. The nanocomposite films were found to be semiconducting, with conductivities in the range 1.13 × 10−4 Sm−1 to 1.06 × 10−3 Sm−1. The presence of non-fluorescent H-aggregate in the nanocomposite film samples is also discussed. The nanocomposite films exhibit low threshold NLO properties, as studied using the Z-scan technique. These samples show negative nonlinear refractive coefficients, n2 of the order of 10−6 cm/W. The optical limiting of 632.8 nm CW He–Ne laser light, exhibited by TBO-PVA nanocomposite films is also demonstrated. The estimated third order nonlinear parameters as well as limiting threshold values are compared to or much better than those reported ones in the literature. Overall, these interesting observations bring into focus that this new low-cost, semiconducting NLO material as a smart material can be capable for developing future photonic and optoelectronic devices such as OLEDs, optical limiters, optical switches, sensors, etc.
References
Camargo PHC, Satyanarayana KG, Wypych F (2009) Nanocomposites: synthesis, structure, properties and new application opportunities. Mat Res 12:1–39
Zhang W, Zhao YS (2016) Organic nanophotonic materials: the relationship between excited-state processes and photonic performances. Chem Commun 52:8906–8917
Mallakpour S, Sadeghzadeh R (2017) Surface modification of alumina with biosafe molecules: nanostructure, thermal, and mechanical properties of PVA nanocomposites. J Appl Polym Sci 134(10):44561
Mittal V (2012) Characterization techniques for polymer nanocomposites. Wiley, Hoboken
Zhang Y, Yang J (2013) Design strategies for fluorescent biodegradable polymeric biomaterials. J Mater Chem B 1:132–148
Schibilla F, Stegemann L, Strassert CA, Rizzo F, Ravoo BJ (2016) Fluorescence quenching in β-cyclodextrin vesicles: membrane confinement and host–guest interactions. Photochem Photobiol Sci 15:235–243
Boreddy SRR (2011) Advances in nanocomposites-synthesis, characterization and industrial applications. InTech, London
Gnana Kumar G, Kee Suk Nahm (2011). Polymer nanocomposites—fuel cell applications, advances in nanocomposites—synthesis, characterization and industrial applications. In: Dr. Reddy B (ed). ISBN: 978-953-307-165-7, InTech. http://www.intechopen.com/books/advances-in-nanocompositessynthesischaracterization-and-industrial-applications/polymer-nanocomposites-fuel-cell-applications
Dancus I, Vlad VI, Petris A, Frunza S, Beica T, Zgura I, Nicolescu FA, Jerca VV, Vuluga DM (2010) Low power laser induced optical nonlinearities in organic molecules. Roman Rep Phys 62(3):567–580
Lee KJ, Oh JH, Kim Y, Jang J (2006) Fabrication of photoluminescent-dye embedded poly(methyl methacrylate) nanofibers and their fluorescence resonance energy transfer properties. Adv Mater 18:2216–2219
Sreekumar G, Frobel PGL, Muneera CI, Sathiyamoorthy K, Vijayan C, Mukherjee C (2009) Saturable and reverse saturable absorption and nonlinear refraction in nanoclustered Amido Black dye–polymer films under low power continuous wave He–Ne laser light excitation. J Opt A Pur Appl Opt 11:125204
Frobel PGL, Suresh SR, Mayadevi S, Sreeja S, Mukherjee C, Muneera CI (2011) Intense low threshold nonlinear absorption and nonlinear refraction in a new organic–polymer nanocomposite. Mater Chem Phys 129:981–989
Sreeja S, Sreedhanya S, Smijesh N, Philip R, Muneera CI (2013) Organic dye impregnated poly(vinyl alcohol) nanocomposite as an efficient optical limiter: structure, morphology and photophysical properties. J Mater Chem C 1:3851–3861
Sreedhar S, Illyaskutty N, Sreedhanya S, Philip R, Muneera CI (2016) An organic dye-polymer (phenol red-poly(vinyl alcohol)) composite architecture towards tunable -optical and -saturable absorption characteristics. J Appl Phys 119:193106
Cerroni B, Chiessi E, Margheritelli S, Oddo L, Paradossi G (2011) Polymer shelled microparticles for a targeted doxorubicin delivery in cancer therapy. Biomacromol 12(3):593–601
D’mello AXP, Sylvester TV, Ramya V, Britto FP, Shetty PK, Jasphin S (2016) Metachromasia and metachromatic dyes: a review. Int J Adv Health Sci 2:12–17. https://www.ijahs.net/uploads/2/6/7/7/26772457/03_ijahs_2_10__02_ra.pdf
Sridharan G, Shankar AA (2012) Toluidine blue: a review of its chemistry and clinical utility. J Oral Maxillofac Pathol 16:251
Ganguly B, Nath RK, Panda AK (2013) Spectral studies on the interaction of toluidine blue O with bovine serum albumin. J Surface Sci Technol 29:1–16
Viswanath Varsha, Muneera CI (2017) Nonlinear refraction behaviour of toluidine blue O Dye-PVA nanocomposite films under CW laser light excitation. Mater Today Proc 4(2):4412–4416
Rekha RK, Ramalingam A (2007) Workshop on physics and technology of all-optical communication components and devices department of physics and meteorology. IIT Kharagpur, Kharagpur
Lucio D’Ilario and Andrea Martinelli (2006) Toluidine blue: aggregation properties and structural aspects. Model Simul Mater Sci Eng 14:581–595
Cesca T, Calvelli P, Battaglin G, Mazzoldi P, Mattei G (2012) Local-field enhancement effect on the nonlinear optical response of gold-silver nanoplanets. Opt Exp 20:4537–4547
Kiran PP, Srinivas NKMN, Reddy DR, Maiya BG, Dharmadhikari A, Sandhu AS, Kumar GR, Rao DN (2002) Heavy atom effect on nonlinear absorption and optical limiting characteristics of 5,10,15,20-(tetratolyl) porphyrinato phosphorus (V) dichloride. Opt Commun 202:347–352
Henari FZ (2001) Optical switching in organometalic phthalocyanine. J Opt A Pure Appl Opt 3:188–190
Seven KM, Cogen JM, Gilchrist JF (2016) Nucleating agents for high-density polyethylene—a review. Polym Eng Sci 56:541–554
Sheik-Bahae M, Said A, Wei T, Hagan D, Stryland E (1990) Sensitive measurement of optical nonlinearities using a single beam. IEEE J Quantum Electron 26:760–769
Zelinschi CB, Stoica I, Dorohoi DO (2014) Changes in morphology and optical properties of polyvinyl alcohol foils induced by Congo red dye concentration and stretching degree. J Polym Eng 34(4):345–351
Yang CC (2006) Study of alkaline nanocomposite polymer electrolytes based on PVA–ZrO2–KOH. Mater Sci Eng B 131:256–262
Roy D, Das NM, Shakti N, Gupta PS (2014) Comparative study of optical, structural and electrical properties of zinc phthalocyanine Langmuir-Blodgett thin film on annealing. RSC Adv 4:42514–42522
Thomas S, Mohammed AP, Gowd EB, Kalarikkal N (2018) Crystallization in multiphase polymer system. Elsevier, Amsterdam
Usacheva MN, Teichert MC, Biel MA (2003) The role of the methylene blue and toluidine blue monomers and dimers in the photoinactivation of bacteria. J Photochem Photobiol B 71:87–98
Sakho EM, Oluwafemi OS, Perumbilavil S, Philip R, Kala MS, Thomas S, Kalarikkal N (2016) Rapid and facile synthesis of graphene oxide quantum dots with good linear and nonlinear optical properties. J Mater Sci Mater Electron 27:10926–10933
Esfahani ZH, Ghanipour M, Dorranian D (2014) Effect of dye concentration on the optical properties of red-BS dye-doped PVA film. J Theor Appl Phys 8:117–121
Elashmawi IS, Abdelrazek EM (2010) Role of CoBr 2 on the structural, optical and magnetic properties of polyvinyl alcohol films. J Appl Polym Sci 115:2691–2696
Chen Y, Hanack M, Araki Y, Ito O (2005) Axially modified gallium phthalocyanines and naphthalocyanines for optical limiting. Chem Soc Rev 34:517–529
Belušáková S, Lang K, Bujdak J (2015) Hybrid systems based on layered silicate and organic dyes for cascade energy transfer. J Phys Chem C 119:21784–21794
Głowacki ED, Leonat L, Irimia-Vladu M, Schwödiauer R, Ullah M, Sitter H, Bauer S, Sariciftci NS (2012) Intermolecular hydrogen-bonded organic semiconductors—quinacridone versus pentacene. Appl Phys Lett 101:023305
Kumar GV, Chandramani R (2010) Doping and irradiation dependence of electrical conductivity of Fe3+ and Ni2+ Doped polyvinyl alcohol films. Acta Phys Pol A 117:917–920
Kilarkaje S, Manjunatha V, Devendrappa H (2011) Optical and electrical characterization of (peo1methyl violet) polymer electrolytes. J Appl Polym Sci 124:2558–2566
Alias AN, Zabidi ZM, Ali AMM, Harun MK, Yahya MZA (2013) Optical characterization and properties of polymeric materials for optoelectronic and photonic applications. Int J Appl Sci Technol 3(5):11–38
Srikanth C, Sridhar CB, Nagabhushana BM, Mathad RD (2014) Characterization and DC conductivity of novel CuO doped polyvinyl alcohol (PVA) nano-composite films. Int J Appl Innov Eng Manag 3:200–208
Deng X, Zhang X, Wang Y, Song Y, Liu S, Li C (1999) Intensity threshold in the conversion from reverse saturable absorption to saturable absorption and its application in optical limiting. Opt Commun 168:207–212
Kim Wihan, Applegate Brian E (2015) In vivo molecular contrast OCT imaging of methylene blue. Opt Lett 40:1426–1429
Cassano T, Tommasi R, Meacham AP, Ward MD (2005) Investigation of the excited-state absorption of a Ru dioxolene complex by the Z–scan technique. J Chem Phys 122:154507
Vivas MG, Fernandes EGR, Rodríguez-Méndez ML, Mendonca CR (2012) Study of singlet excited state absorption spectrum of lutetium bisphthalocyanine using the femtosecond Z-scan technique. Chem Phys Lett 531:173–176
Zhu XiaoRong, Sun ZhenRong, RuiMingNiu HePingZeng, Wang ZuGeng, Lang JianPing, Xu Z, Li R (2003) Optical nonlinear properties and optical limiting for [(n-Bu)4N]4 [MoS4Cu]4. J Appl Phys 94:4772–4775
Rao SV, Rao DN, Akkara JA, DeCristofano BS, Rao DVGLN (1998) Dispersion studies of non-linear absorption in C using Z-scan. Chem Phys Lett 297:491–498
Pramodini S, Deepika Sandhya, Rao Ashok, Poornesh P (2014) Studies on thermally induced third-order optical nonlinearity and optical power limiting response of Azure B under CW He–Ne laser excitation. Opt Laser Technol 62:58–62
Gao Y, Zhang X, Li Y, Liu H, Wang Y, Chang Q, Jiao W, Song Y (2005) Saturable absorption and reverse saturable absorption in platinum nanoparticles. Opt Commun 251:429
Rekha RK, Ramalingam A (2009) Non-linear characterization and optical limiting effect of carmine dye. Indian J Sci Technol 2:27–31
Jaffar AF (2013) Solvent effect on the third order nonlinearity of oxazine Dye doped PMMA films by using Z-scan techniques. Int J Adv Res Technol 2:56–64
He T, Wang C (2008) The study on the nonlinear optical response of Sudan I. Opt Commun 281:4121–4125
Yin M, Li H, Tang S, Ji W (2000) Determination of nonlinear absorption and refraction by single Z-scan method. Appl Phys B Lasers Opt 70:587
Tripathy U, Bisht PB (2006) Simultaneous estimation of optical nonlinear refractive and absorptive parameters by solvent induced changes in optical density. Opt Commun 261:353–358
Mian SM, McGee SB, Melikechi N (2002) Experimental and theoretical investigation of thermal lensing effects in mode-locked femtosecond Z-scan experiments. Opt Commun 207:339–345
Mathews SJ, Kumar SC, Giribabu L, Rao SV (2007) Large third-order optical nonlinearity and optical limiting in symmetric and unsymmetrical phthalocyanines studied using Z-scan. Opt Commun 280:206–212
Sarkhosh L, Aleali H, Karimzadeh R, Mansour N (2010) Large thermally induced nonlinear refraction of gold nanoparticles stabilized by cyclohexanone. Phys Status Solidi A 207:2303–2310
Mathews SJ, Kumar SC, Giribabu L, Rao SV (2007) Nonlinear optical and optical limiting properties of phthalocyanines in solution and thin films of PMMA at 633 nm studied using a CW laser. Mater Lett 61:4426–4431
Vivien L, Lanςon P, Riehl D, Hache F, Anglaret E (2002) Carbon nanotubes for optical limiting. Carbon 40:1789–1797
Al-Nasir EA, Al-Ahmad AY, Hussein AA, Ali QM, Sultan AA, Al-Mowali AH (2013) Low optical limiting and nonlinear optical properties of vanadyl phthalocyanine using a CW laser. Chem Mater Res 3:18–26
Sathiyamoorthy K, Vijayan C, Kothiyal MP (2008) Low power optical limiting in ClAl-Phthalocyanine due to self defocusing and self phase modulation effects. Opt Mater 31:79–86
Acknowledgements
The author Varsha Viswanath thanks Council of Scientific & Industrial Research-National Institute for Interdisciplinary Science and Technology (CSIR-NIIST), Trivandrum, Kerala, India for the valuable support in providing UV–visible absorption spectra; and National Centre for Earth Science and Studies (NCESS), Trivandrum, Kerala, India for the valuable support in providing X-Ray diffraction (XRD) patterns. The author Varsha Viswanath also thanks Mahatma Gandhi University, Kottayam and Sophisticated Test and Instrumentation Centre (STIC), Cochin for the valuable support in providing High Resolution Transmission Electron Microscopic (HRTEM) analysis.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Viswanath, V., Nair, S.S., Subodh, G. et al. Emission features, surface morphology and optical limiting properties of semiconducting Toluidine Blue O dye-poly(vinyl alcohol) nanocomposite architecture. SN Appl. Sci. 1, 43 (2019). https://doi.org/10.1007/s42452-018-0043-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-018-0043-6