Abstract
The aim of this study was to evaluate and characterize the hydrogeochemistry and quality of groundwater (for human consumption) in Ogbaru district, southeast Nigeria. Borehole samples were subjected to physicochemical, bacteriological, hydrogeochemical, and statistical analysis. The physicochemical characteristics of the water were below standard maximum permissible limits for drinking water. Moreover, heavy metals were found in low concentrations (below their permissible limits) in all of the samples. However, majority of the samples have pH values below the allowable limits of 6.5–8.5, indicating they are slightly acidic waters. The dominance of cations and anions is in the order: Ca > Mg > Na > K and HCO3 > Cl > NO3 > SO4 > PO4 > NO2, respectively. Mg–Ca–HCO3 water type dominated the area, constituting about 47.4% of the total samples. Ca–Mg–HCO3 water type constituted about 16%; Mg–Ca–Na and Ca–Mg–Na–HCO3 water types constituted 10.5% each, whereas Ca–Mg–Cl, Mg–Ca–Cl–NO3, and Ca–Mg types made up 5.2% each. The possible sources and influencers (both anthropogenic and geogenic) of the physical and chemical water quality parameters were identified by correlation and principal component analyses. Although most of the groundwater samples are of good quality based on the physicochemical properties, the presence of coliforms indicates that their quality is questionable and hence not safe for drinking. Therefore, treating them before use is recommended.
Similar content being viewed by others
1 Introduction
The use of water for various purposes largely depends on its quality. Groundwater resources in many countries are alternative sources of water for homes, industries, and farms, owing to the increasingly worrisome levels of contamination and pollution of surface water resources. In developing countries, access to safe water for drinking and domestic purposes by inhabitants is a major crisis governments and individuals fight against [1]. Contamination or pollution of groundwater resources is a threat to the quality and sustainability of available drinking water and public health [2, 3].
Sources of groundwater contaminants span from homes to industries to farms. In agricultural areas, shallow groundwater systems are more vulnerable to contamination and pollution, after surface waters [4,5,6]. Nutrients in forms of NO3, PO4, and K are common agricultural contaminants in such areas [7]. Other oxides and heavy metals can also be found in excess. However, the level of the impacts of contaminants and pollutants on groundwater resources is dependent on several factors including nature and composition of contaminants, source and quantity of contaminants or pollutants, mobility and solubility of contaminant, land use/land cover, biological activities, climate conditions, geology, topography, distribution of surface water networks recharging the aquifers, hydrogeological conditions, direction and rate of underground water movement, etc.
In order to establish sustainable development and management of groundwater resources, it is pertinent to regularly assess and monitor their quality, on the basis of physical, chemical, and microbial makeups. Identification of contaminated waters in an area assists in the rationalization of sampling and designing of pollution monitoring programs [8].
Hydrogeochemistry is an important subject required in the monitoring, assessment, and management of water resources. Hydrogeochemical facies is an important diagnostic chemical aspect of groundwater solutions occurring in hydrologic systems and provides information on the distinct zones of cations and anions distributions along different layers of various aquifer systems and how the elemental fluxes are exchanged through atmospheric, terrestrial, and aquatic interactions [9,10,11,12,13]. Trace elements’ mobility and occurrence in groundwater are strongly influenced by adsorption on clay minerals, groundwater pH and redox conditions, organic matter, and other crystalline and amorphous substances that make up the porous media; thus, their concentrations in groundwater are controlled by geochemical processes [14,15,16]. Interactions between the environment and rocks/soils influence the chemistry of water. The ions distributed in water are pointers to its type and quality. An understanding of the geochemical components in water, as well as the physical ones, is important in determining its origin and suitability for drinking, domestic, industrial, and irrigation purposes [8, 16, 17]. Also, the microorganisms in water resources play significant role in defining their quality or class [10, 18].
Literature reporting on the hydrogeochemical facies, water types, and groundwater quality of the intense agricultural areas in Ogbaru is scarce. However, Ekenta et al. [19] investigated the hydrogeological and aquifer characteristics of the Ogbaru district and groundwater quality based on the physicochemical and bacteriological properties. But, their research did not report on the hydrogeochemical characteristics and evolution of the groundwater in this agricultural area. Therefore, this study was designed to use a more sophisticated approach in: (1) identifying the physicochemical properties of the waters, (2) identifying the total coliform (TC) and fecal coliform (FC) counts in the waters, (3) identifying the water facies, water types, and the influencers of the hydrogeochemical characteristics of the study area, and (4) assessing the suitability of the waters for drinking purposes. This study provides essential information needful in monitoring, management, and sustainability of the groundwater resources for human health and economic development in the Ogbaru district.
2 Description of the study area
2.1 Location, geomorphology, and climate
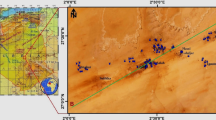
Ogbaru district is situated at the bank of Niger River in southeastern Nigeria and characterized by several wetlands/swamps. It lies within latitudes 5°42′N to 6°10′N and longitudes 6°41′E to 6°50′E (Fig. 1) and known for agricultural activities. The relief is a lowland of heights ≤ 50 m above the sea level [19]. There are several rivers, streams, ponds, and creeks in the area that generally pay tribute to Niger River, which in turn empties into the Atlantic Ocean. Most parts of the area form the flood/alluvial plains of the Niger River. Because of the abundant surface water bodies in the area, farming and fishing are common occupations for the inhabitants. Dry and rainy seasons prevail in the area. The former is experienced between October–March, and the latter, April–September. The natural vegetation in the area is tropical rainforest, though irregular deforestation and agricultural activities influence the vegetation cover. The annual rainfall is high, averaging between about 1800 and 2300 mm [12, 13]. The area is highly humid, especially during rainy seasons. Average relative humidity in the area is about 26% per annum. Average annual temperature is within the range of 24–28 °C in the day, but can be as low as 16–18 °C in the night.
2.2 Geology and hydrogeology
Ogbaru district is majorly underlain by alluvium deposit, though the Benin and Ogwashi formations underlie smaller parts of the district (Fig. 1). Among the stratigraphic units, the Ogwashi formation is the oldest whereas the alluvium deposit is the youngest. Because of the swampy nature of the study area, alluvial muds dominate, leaving the surface waters almost polluted with sediments. The borehole depths in the area are shallow and vary from 5 to 50 m; the static water levels vary from 1.89 to 23.14 m [19, 20]. The values of geophysical and hydraulic parameters indicate that the aquifers are characteristic of silty and clean sand materials [20, 21]. However, the main aquifers in the area are alluvial sands [19]. The presence of numerous surface water bodies in the area suggests that they are influents, providing ready recharge systems for the aquifers.
3 Research methodology
3.1 Water sampling
In order to achieve the objectives of this research, a total of 19 borehole samples were randomly collected with 1-l plastic bottles and analyzed for physicochemical parameters and bacteriological counts. The sample bottles were sterilized with concentrated hydrochloric acid and rinsed with distilled water. At respective sampling stations, sample water was also used to rinse the bottles. The sample bottles were well labeled, sealed, and placed in ice-crested coolers before they were taken to the laboratory for analysis. The refrigeration was to prevent the samples from reacting with the sample bottles or other external factors.
3.2 Field and laboratory analysis
The reagents used in this work were analytical grade chemicals (i.e., high purity reagents suitable for analytical procedures, Merck Grade). Physicochemical parameters of all the water samples were analyzed according to the standard procedures prescribed by the American Public Health Association (APHA) [22]. The pH, temperature, total dissolved solids (TDS), and electric conductivity (EC) were measured in the field. The electrical conductivity (EC) and total dissolved solids of the water samples were determined using a Mettler Toledo conductivity meter. Parameters analyzed in the laboratory include salinity, turbidity, total hardness (TH); NO3, NO2, PO4, SO4, HCO3, Cl, Na, K, Mg, Ca; total counts (TC), and fecal counts (FC). Analyzed heavy metals are Fe, Zn, Cu, Cd, Cr, Ni, and Mn. Heavy metals and cation concentrations in the samples were analyzed using atomic absorption spectrophotometric (AAS) technique (Bulk Scientific 210 VGP), while the anions were analyzed using ion chromatographic method.
Microbial analysis of the samples, which employed the pour plate technique (using heterotrophic plate count), was targeted at detecting and enumerating the total coliforms and fecal coliforms present. Each sample was diluted to a factor of 10−3, and 1 ml of the diluent was plated on nutrient agar in triplicates [23, 24]. The analysis was conducted in triplicates for each of the samples to ensure accuracy. The plates were inverted and incubated at 37 °C for 24–48 h under aerobic condition. The total viable count (TVC) was recorded in colony forming units per ml (cfu/ml).
3.3 Data analysis and interpretation
Classification diagrams, including Piper and Durov diagrams, were produced using AquaChem software (v. 2014, Schlumberger Water Services, Canada). This aided in the identification of the hydrogeochemical facies dominating the area. Microsoft Excel (v. 2013) was used in producing charts. In addition, a multivariate correlation and principal component analysis of physicochemical parameters and heavy metals tested in the samples were done using correlation coefficients at the two-tailed test of significance (0.05 and 0.01 levels) with the aid of SPSS software (v. 22, IBM Inc.). Furthermore, the potability of the water resources was evaluated by comparing obtained results with the World Health Organization (WHO) [25] maximum allowable limits for water, as well as with those of the Nigerian Standards for Drinking Water Quality (NSDWQ) [26].
4 Results and discussion
4.1 Descriptive summary of the physicochemical characteristics
Table 1 shows the statistical summary of all the analyzed parameters and their comparisons with the WHO [25] and NSDWQ [26] standards. There were variations in temperature values among the water samples, ranging from 25 to 29 °C. Majority of the samples had pH values not within the maximum allowable limits of WHO and NSDWQ (Table 1, Fig. 2). The pH results indicate that the groundwater samples are slightly acidic to neutral. The pH of water has no direct effect on human health [25]. However, it usually has a link with other chemical constituents in water [10, 27]. EC, TDS, and salinity values in the samples ranged from 10 to 320 µS/cm, 20 to 140 mg/l, and 5 to 78 mg/l, respectively. These values were far below allowable limits of WHO and NSDWQ. EC is an indicator of the presence of ions and concentrations of dissolved components. In this study, it was observed that the TDS and EC values were not proportional. The distribution and relationship between the TDS and EC values are shown in Fig. 3. According to a TDS classification proposed by Fetter [28], all the groundwater samples belonged to fresh water category (TDS < 1000 mg/l). The low salinity of the waters indicates that they had no links with salty connate water or seawater recharge systems. It also indicates that the area has no record of drought or extreme evaporation. Also, it could be that the characteristic high rainfall in the area effectively annuls the impact of excess accumulations of salt ions (Na+ and Cl−) in the groundwater.
Turbidity values were also observed to be below maximum permissible limits of 5NTU. This affirms that dissolved solids in the waters were minimal and that microorganisms would find it difficult to multiply or resist disinfection [29]. Three samples (BH04, BH11, BH17) recorded TH values above the WHO desirable limit of 100 mg/l. Others were well below the limit (100 mg/l; Table 1). According to water classification proposed by Todd [30] on the basis of total hardness, most of the groundwater samples are soft to moderately hard (Table 2). This implies that the users less likely risk kidney stone associated with the use of waters that have high hardness values. Also, the ability of soaps to produce lather in the soft waters will not reduce and the boiling point of the waters will not increase.
NO3, NO2, and PO4 values ranged from 2 to 36 mg/l, 0 to 0.05 mg/l, and 0 to 1.2 mg/l, respectively. Nitrate and phosphate are the major plant nutrients from fertilizers. The presence of high nitrate concentration in drinking water increases the incidence of gastric cancer and other potential health hazards to infants and pregnant women [30,31,32]. Nitrate leaching is enhanced by high infiltration potential of soil layer and low runoff potential. Their concentrations are influenced by soils, climate and atmospheric inputs, local geology, topography, and human activities related to land use [33]. According to Cushing et al. [34], the concentration of nitrate does not exceed 10 mg/l in water under natural conditions. However, these values are below the WHO and NSDWQ limits (Table 1). Even though the groundwater samples were not nutrient polluted, the upscaling values of NO3 suggest that agricultural activities (use of fertilizers) in the area influence their concentration in the groundwater. Samples BH01, BH02, BH04, BH08, and BH16 are more vulnerable to nutrient pollution.
SO4, Cl, and HCO3 results varied, respectively, from 0 to 15 mg/l, 0 to 47 mg/l, and 11 to 158 mg/l. These values were within the permissible limits of WHO and NSDWQ. Low concentrations of SO4 in the waters could be attributed to the absence of sulfate-rich rocks like gypsum in the area. In addition, the low sulfate content confirms that the study area was not an industrial city where higher concentrations are possible, due to industrial processes and emissions. Concentrations of Cl could be attributed to domestic wastes, poor sanitary conditions, leaching from soil layers, or natural geochemical processes [10]. The bicarbonates were probably derived from weathering of silicate rocks and minerals (feldspars) by carbonic acid, atmospheric and soil CO2 gas, and/or the reaction of NO3− and SO42− by organic matter [33, 35].
K, Na, Mg, and Ca contents were well below the maximum desirable limits of WHO and NSDWQ [25, 26]. Their mean values are 1.08 mg/l, 8.89 mg/l, 22 mg/l, and 35.84 mg/l, respectively. Low concentrations of the cations are attributed to the less abundance of rocks rich in them. Na and Ca concentrations in the waters could be from plagioclase feldspar dissolution. Also, the low concentrations of Na seem to correlate well with the low salinity of the samples. Similarly, although not proportionally distributed, low Mg and Ca concentrations point to why the TH values were generally low (< 100 mg/l). Figure 4 shows the disproportionality between the TDS, TH, Mg, and Ca concentrations in the samples.
The groundwater samples recorded no excess concentration of the analyzed heavy metals. Cu, Cd, Cr, and Ni were not detected or occurred in infinitesimal concentrations in all the water samples. Only four samples (BH04, BH07, BH14, BH19) recorded Zn concentrations, ranging from 0.01 to 0.02 mg/l. Values of Fe and Mn concentrations ranged from 0 to 0.2 mg/l and 0 to 0.09 mg/l, respectively. All the obtained values are below the limits set by the WHO and NSDWQ (Table 1).
4.2 Bacteriological counts in groundwater
The presence of some (usually pathogenic) microorganisms in water is a threat to its quality. Quality and safe water are usually characterized by zero coliform content. Total coliform counts in the groundwater samples ranged from 1 to 7 cfu/ml, exceeding the 0 cfu/ml limit by WHO [25, 29] but below the 10 cfu/ml limit by NSDWQ [26] (Table 1). Majority of the samples met the fecal coliform count standard (0 cfu/ml) of WHO and NSDWQ. Only samples BH08, BH15, and BH18 recorded 1 cfu/ml, exceeding the maximum allowable limit.
4.3 Hydrogeochemical characteristics
4.3.1 Water types and facies
A trilinear diagram proposed by Piper [36] is very useful in understanding the hydrogeochemical regime of an area. The percentages of cations and anions, expressed in meq/l, in water are plotted in the diagram (Fig. 5) to show the similarities and differences among the water samples. For the analyzed groundwater samples, the dominance of major cations and anions is in the order: Ca > Mg > Na > K and HCO3 > Cl > NO3 > SO4 > PO4 > NO2, respectively. The general overview of the hydrogeochemical characteristics of the water samples is shown in Table 3.
Ca, Mg, and HCO3 are the dominant cations and anion occurring in the waters. Hence, the area is dominated by alkaline earth-bicarbonate groundwater facies. This confirms that the groundwater has more alkaline earth metals (Ca + Mg) than alkali metals (Na + K) and more of weak acids (HCO3 + CO3) than strong ones (SO4 + Cl). The concentration of HCO3− in water may be due to decomposition of organic matter.
Alkalinity is simply defined as a water’s capacity to resist changes in pH that would make it become more acidic. In this study, the groundwater samples are generally characterized by secondary alkalinity. This implies that the waters’ buffering capacity is a function of non-carbonate rock (weathering) origin. In other words, it can be inferred that the evolution of the alkalinity of the groundwater samples is due to upscale concentration of HCO3, which in turn influences carbonic acid weathering. Moreover, the majority of the samples are soft waters (on the basis of total hardness, TH). This is confirmatory of the alkalinity of the groundwater samples. Generally, soft waters are often characterized by low-to-moderate alkalinity. This has the potency of predisposing them to frequent pH fluctuations.
The various water types in the study area were revealed by the AquaChem software. The water types were confirmed by plotting the concentrations of major cations and anions for individual samples on stiff and radial diagrams (not shown) and are summarized in Table 3. Seven water types characterize the study area. It was observed that Mg–Ca–HCO3 water type constituted 47.4%, dominating the area. Ca–Mg–HCO3 water type constituted about 16%; Mg–Ca–Na and Ca–Mg–Na–HCO3 water types constituted 10.5% each, whereas Ca–Mg–Cl, Mg–Ca–Cl–NO3, and Ca–Mg water types each made up 5.2%. The water types dominating this wetland area confirm that the waters have no links to seawater intrusion process, as waters that have mixing with seawater are dominantly composed of alkalis (Na + K) and strong acids (Cl + SO4) [35].
The major ions of the groundwater samples were also plotted in a Durov diagram (Fig. 6) because it aids in depicting evolutionary and hydrogeochemical trends and processes taking place in groundwater systems. The Durov diagram confirmed the distinctions shown in the cation and anion fields of the Piper diagram. The samples generally seem to have a uniform (similar) geochemical trend. Majority of the groundwater samples are plotted in Ca + Mg-rich cation field and HCO3 + CO3-rich anion field. This indicates that there exists an ionic exchange of Ca–Mg–HCO3-rich waters, evolving from possible silicate hydrolysis, cation exchange, or a combination of both [37]. For clarity purposes, the silicate hydrolysis and ionic exchange are discussed in a different subsection. None of the samples appeared in the pH field, confirming the waters are mostly slightly acidic.
4.3.2 Evaluating hydrogeochemical evolution using Gibbs mechanism
It was necessary to examine the water–environment interactions that give the waters their characteristic chemistry. Gibbs [38] diagram helps in establishing the relationships between water chemistry and hydrologic processes and lithology of an aquifer. Although the Gibbs diagram was originally drawn for surface water, many researchers [12, 32, 35, 39,40,41,42,43] have used it in groundwater studies. The area under study is characterized by shallow aquifers, swamps, and streams/rivers. It is, therefore, believed that there are possible constant interactions between the groundwater systems and the surface water bodies. Table 4 shows the samples’ Gibbs ratios (for cations and anions), and Fig. 7 presents Gibbs diagrams used in assessing this relationship among the samples. Dominance due to precipitation, evaporation, and rock–water interaction (weathering) is three distinct fields of the Gibbs plot. Results show that hydrogeochemical properties of the groundwater samples are controlled by rock–water interactions. The chemistry of the groundwater samples is largely influenced by chemical weathering of rock-forming minerals and anthropogenic activities. The rock–water interaction is controlled by factors such as relief, climate, and chemical composition of rocks and soils in the area [38, 44]. The rocks and soils in the study area are far more enriched in Ca and Mg than the other cations (Na + K). Calculated Ca/Mg ratios, as well as SO4/Cl ratios, show that the groundwater systems are typically recharged from freshwater sources (Table 5). However, most of the samples have TDS values below 100 mg/l.
4.3.3 Silicate hydrolysis and ion exchange
So far, it has been revealed that the hydrogeochemistry of the groundwater systems is influenced by the hydrogeological setting, geology, chemical weathering, ionic exchange, and agricultural inputs. As cited earlier, the dominant water facies is Ca–Mg–HCO3. From the Gibbs analysis, rock–water interaction (weathering) is a dominant process in the hydrogeochemical evolution of the groundwater samples. Hence, the influence of silicate hydrolysis and ion exchange on the overall geochemistry of the waters will be expatiated.
In chemical weathering, hydrolysis is a very important chemical change that takes place. Hydrogen ions in water react with minerals and cause them to disintegrate into various ions. This reaction is often accelerated by the presence of dissolved CO2 which can also be derived from respiration and decay of organisms in the soil, to yield carbonic acid, H2CO3 [45].
The study area is dominated by silicate rocks and minerals. The silicate weathering process is well supported by a high (sufficient) concentration of HCO3 [39]. However, a hydrogen ion is known to be the most potent agent of hydrolytic weathering processes. Therefore, from Eq. 2, it can be seen that when more hydrogen ions, alongside carbonic acid, are liberated into the aquiferous system, more dissolution of silicate minerals will be enhanced. However, the liberation of excess H+ than OH− will make the groundwater samples far more acidic, with pH far less than 5 [28]. Since sands and alluviums are the dominant geologic units in the study area, silica and feldspars are consequently the dominant minerals. Feldspars are rich in calcium, sodium, and potassium. In the hydrolysis of these minerals, carbonic acid and water alter them to insoluble clay residue and dissolved ions [45]. The clay residues constitute the components increasing the turbidity of water, whereas the dissolved ions influence other physical properties (including alkalinity and EC) of the water.
A (Ca + Mg) versus (SO4 + HCO3) scatter plot (Fig. 8) confirms that silicate weathering dominates the study area more than carbonate weathering [13, 39, 46]. The concentration of calcium ions in the groundwater samples is relatively higher when compared to that of magnesium ions (Table 5). The magnesium concentration could be due to the weathering of magnesium minerals, whereas calcium might have been leached from weathering of calcic minerals (plagioclase feldspars in sands and alluviums) or cation exchange process. However, carbonic acid dissolution is an important process believed to expedite the silicate weathering which releases calcium ions into the groundwater samples (Eqs. 3 and 4).
The Na+ is believed to come from the dissolution of sodium-bearing minerals (e.g., sodic plagioclases like albite). According to Meybeck [47] and Kumar et al. [39], Na/Cl ratio greater than 1 typically indicates that sodium ions were derived from silicate weathering. However, the result showed that three samples have Na/Cl ratio greater than 1, whereas the others were less than 1 (Table 5), suggesting that there could be a significant reduction of Na concentration due to ion exchange process (Eq. 5). However, it has been reported that reverse cationic exchange takes place with sodium reducing the concentrations of Ca2+ and Mg2+ ions [13, 33], when sources rich in sodium are more abundant than those of calcium and magnesium. If the halite dissolution process was to be responsible for the sodium concentration in the samples, Na/Cl ratio could be approximately equal to 1 [39, 47].
Low concentrations of sodium compared to calcium indicate the absence of readily soluble sodic minerals (plagioclase) or the action of base exchange where sodium has been exchanged for calcium as shown in Eq. 6. Generally, this trend is accounted for by cation exchange reactions that occur in favor of calcium as the water flows through calcium-rich rock/soil units of the aquifer system.
As the water flow reaches sodic minerals, the reaction path shown in Eq. 7 may be followed, for the water to acquire Na+:
Likewise, potassium, a common component of fertilizers (rich in NPK) and thus believed to be derived from agricultural sources, can also be leached from dissolution of orthoclase feldspars in the presence of carbonic acid and water (Eq. 8):
4.4 Multivariate statistical analysis of hydrogeochemical data
4.4.1 Correlation coefficient matrix
The Pearson’s correlation matrix analysis is a useful tool that can indicate the origins and associations between hydrogeochemical parameters [12, 13, 33, 35]. For this study, the strength of the relationships between all the parameters is shown in Table 6. Negative and negligible relationships exist between temperature and other parameters. This is indicative that temperature has little or no control over the accumulations of the cations and anions in the area [12]. Many of the parameters have weak positive correlations with one another. Ca appears to have the highest positive (weak) association with others. The weak positive associations among many of the parameters suggest that many factors could be influencing their occurrence in the groundwater samples. It could also mean that the direct influence each of them has on one another is positively minimal. However, moderate positive correlation exists between EC and TDS (0.503), pH and turbidity (0.534), PO4 and EC (0.554), Cl and NO3 (0.535), and Fe and TH (0.653). This suggests that each of the pairs significantly influences the occurrence (concentration) of their respective counterparts.
A strong positive relationship (0.926) exists between the nutrients, PO4 and NO3, in the waters. Also positive correlations exist between K, NO3, and PO4 (Table 6). This indicates that their occurrence is possibly linked to the use of fertilizers (NPK) in the area [17]. In this study, Mn has a positive correlation with NO3, indicating possible agricultural sources. In agricultural areas, Mn often occurs in water through the leaching of fertilizers over a period of time. A weak negative correlation exists between PO4 and NO2 (− 0.324), indicating that they do not directly influence the occurrence of each other in the waters. Although NO2 is got by the denitrification of NO3 and during the nitrification reaction of ammonium ion (NH4+), the negative correlation between them could be an indicator that their occurrence in the groundwater samples is not proportional. This could mean that there were low numbers of microbes converting nitrates to nitrites. It could also be as a result of other geochemical processes taking place in the waters.
The positive association between EC, pH, and TDS suggests that they probably influence the concentration of major ions in the groundwater samples. A weak negative association (− 0.457) was observed between TH and TDS. The positive linearity between pH and TDS and salinity and TDS is weak. However, the correlation between Mg and salinity and TH is higher than the correlation Ca, K, and Na have with salinity and TH (Table 6). This implies that the salinity and hardness of the waters are more influenced by magnesium salts and minerals than those of the others. The negligible (almost zero) correlation between Na, Mg, and Cl confirms there was no mixing of the groundwater samples with saline water, affirming they were completely recharged by fresh water sources (e.g., ponds, creeks, streams, rivers). It is very likely to find the following correlation pairs (Na and Cl, Mg and Cl, TDS and Cl, Na and TDS, salinity and Na, Cl) in regions where salt deposits or saltwater intrusion interferes with water resource quality [40]. However, in this study, no such association was recorded. Sodium has a very weak negative correlation with chloride (Table 6, Fig. 9). The weak relationship factors between Ca and HCO3 and Ca and SO4 further confirms there was no carbonate rock dissolution and gypsum dissolution, respectively, in the area [13, 40].
4.4.2 Principal component analysis (PCA)
In this study, PCA was used to validate the Pearson’s correlation analysis. Four principal components (PC) were extracted (Table 7). The four components show that the analyzed variables have two distinct sources (anthropogenic and geogenic) influencing their occurrence and consequently the chemical composition of groundwater samples in the study area. The number of significant PC for interpretation was selected with a minimum eigenvalue of 1 [13, 35, 40]. In this study, component (factor) loadings of < ± 0.4 are taken to show poor loadings; ± 0.4 signifies moderate loadings and > ± 0.4 indicates high loadings.
The parameters (EC, TDS, turbidity, NO3, NO2, PO4, HCO3, Na, Mg, and Mn) in PC 1 are typical of origin from the combined influence of geogenic processes (rock–water–environment interactions) and anthropogenic activities [12, 17]. The NO3, NO2, PO4, and Mn are significantly indicative of anthropogenic sources (very likely from agricultural inputs) rather than natural, geogenic processes [12, 17]. In PC 2, the assemblage of parameters (pH, TDS, salinity, SO4, Cl, K, and Ca) is typical of origin from geogenic processes. Likewise, pH, turbidity, HCO3, Na, Mg, Ca, and Fe with high factor loadings in PC 3 indicate geogenic sources. The coexistence of pH and HCO3 in PC 3 could be indicating that the latter influences the former and could also be why the majority of the groundwater samples are slightly acidic [12, 40]. A further shift in the pH (toward acidity) may result in precipitation of CaCO3 from the water [28] (Eqs. 9, 10). Parameters (turbidity, TH, NO3, Ca, Fe, and Zn) in PC 4 are indicative of geogenic processes. However, the NO3 and Zn are linked to an anthropogenic source(s) [12, 17, 35].
Research has shown that the mineralogy of a geological unit (regime) controls, to a large extent, the chemistry and quality of water permeating (flowing) through the system [8, 48]. In line with this thought, it can be deduced that the variables, Mn and Zn, could not be linked to geogenic processes (rock/soil weathering) because the dominant rock/soil types (sands, alluviums, and clays) in the study area are not likely to be composed of such mineralogy [12]. Their possible source could be more from agricultural activities. However, their linkage to geogenic origin could only be possible if there are host rocks associated with siderites and pyrites.
4.5 Groundwater quality and potability assessment
The suitability of groundwater for drinking and domestic purposes was assessed. The results showed that the mean concentrations of the physicochemical parameters in the groundwater samples were generally below the maximum allowable limits for drinking water set by the WHO [25] and NSDWQ [26], indicating that they are generally suitable for drinking and other domestic (cooking, washing, and bathing) purposes. However, all the samples recorded total coliform counts greater than zero, which is contrary to WHO standard, but within the NSDWQ standard. Further, the FC values showed that samples BH08, BH15, and BH18 had fecal contamination. Studies have shown that TC counts in water do not necessarily make the water unsafe for use, and not all FC have hazardous health impact [23]. Although no serious threat to human health is speculated, the counts threaten the quality of the waters for drinking and domestic purposes. Treating (maybe by boiling) the waters before drinking should be encouraged, as this will help improve or maintain their quality.
Irrigation water quality represents the fitness of water for agricultural utility [1, 40]. In order to ascertain whether or not the water samples are good for irrigation purpose, a Wilcox diagram (Fig. 10) was plotted. This diagram plotted for the samples revealed that they are good for irrigation purposes. The suitability of groundwater for irrigation purpose depends on the dissolved ion constituent [33]. EC, sodium absorption ratio (SAR), and salinity are among the critical factors used in assessing the suitability of water for irrigation purpose. High EC value often indicates that water is enriched with salt. This, also, usually makes the water saline, and high salinity leads to the formation of saline soils which does not support plant growth [33]. High sodium concentration (or high salinity) in water poses health hazards (including hypertension) to its users.
5 Conclusions
The groundwater quality and hydrogeochemistry of Ogbaru farming district have been evaluated using an integrated physicochemical, bacteriological, hydrogeochemical, and multivariate statistical study approach. Based on the gross findings of this paper, the following conclusions are drawn:
-
1.
The physicochemical characteristics of the groundwater samples are within the standard maximum permissible limits of the WHO and NSDWQ. Hence, all of the samples are good drinking water. However, the majority of the samples have pH values below the allowable limits of 6.5–8.5, classing the waters as slightly acidic to neutral.
-
2.
The presence of FC in three of the samples and TC in all of the samples showed that they were vulnerable to contamination by more toxic microorganisms. The TC counts were above the WHO standard, though generally below the NSDWQ standard. Although most of the groundwater samples are of good quality based on the physicochemical properties, the presence of coliforms points that their quality is questionable and hence not safe for drinking purposes. Therefore, treating them before use will be advantageous.
-
3.
The predominance of major cations and anions is in the order: Ca > Mg > Na > K and HCO3 > Cl > NO3 > SO4 > PO4 > NO2, respectively. The dominant hydrogeochemical facies is alkaline earth-bicarbonate. Seven water types were identified in this study. Mg–Ca–HCO3 water type constituted 47.4%, dominating the area. Ca–Mg–HCO3 water type constituted about 16%; Mg–Ca–Na and Ca–Mg–Na–HCO3 water types constituted 10.5% each, whereas Ca–Mg–Cl, Mg–Ca–Cl–NO3, and Ca–Mg types made up 5.2% each.
-
4.
The correlation and principal component analyses successfully revealed the interrelationships and possible origins of the analyzed water quality parameters. These statistical tools identified that both anthropogenic (mainly agricultural) and geogenic (chemical weathering and ionic exchange) processes influence the hydrogeochemistry and quality of the drinking groundwater in the study area.
-
5.
The information provided in this study is essential and needful in the monitoring, management, and sustainability of the groundwater resources for public health and economic development in the Ogbaru district. However, further study utilizing more sophisticated approach is recommended.
References
Kalaivanan K, Gurugnanam B, Pourghasemi HR, Suresh M, Kumaravel S (2017) Spatial assessment of groundwater quality using water quality index and hydrochemical indices in the Kodavanar sub-basin, Tamil Nadu, India. Sustain Water Resour Manag. https://doi.org/10.1007/s40899-017-0148-x
Adimalla N (2019) Groundwater quality for drinking and irrigation purposes and potential health risks assessment: a case study from semi-arid region of South India. Expo Health 11(2):109–123. https://doi.org/10.1007/s12403-018-0288-8
Adimalla N, Marsetty SK, Xu P (2019) Assessing groundwater quality and health risks of fluoride pollution in the Shasler Vagu (SV) watershed of Nalgonda, India. Hum Ecol Risk Assess: Int J. https://doi.org/10.1080/10807039.2019.1594154
Hallberg GR (1987) The impacts of agricultural chemicals on ground water quality. GeoJournal 15(3):283–295. https://doi.org/10.1007/BF00213456
Lapworth DJ, Gopal K, Rao MS, MacDonald AM (2014) Intensive groundwater exploitation in the Punjab an evaluation of resource and quality trends. Technical report. NERC Open Research Archive, BGSUK
Purushothaman P, Rao MS, Rawat YS, Kumar CP, Gopal K, Parveen T (2013) Evaluation of hydrogeochemistry and water quality in BistDoab region, Punjab, India. Environ Earth Sci 72:693706. https://doi.org/10.1007/s1266501329929
Keller EA (2011) Environmental geology, 9th edn. Pearson Education, Inc, New Jersey, p 624p
Utom AU, Odoh BI, Egboka BCE (2013) Assessment of hydrogeochemical characteristics of groundwater quality in the vicinity of Okpara coal and Obwetti fireclay mines, near Enugu town, Nigeria. Appl Water Sci 3:271–283
Christopher JD, Robert RR (2005) Definition of hydrochemical facies in the New Zealand national groundwater monitoring programme. J Hydrol 44(2):105–130
Mostafa MG, Uddin SMH, Haque ABMH (2017) Assessment of hydro-geochemistry and groundwater quality of Rajshahi City in Bangladesh. Appl Water Sci 7:4663–4671
Rezaei A, Hassani H (2017) Hydrogeochemistry study and groundwater quality assessment in the north of Isfahan, Iran. Environ Geochem Health. https://doi.org/10.1007/s10653-017-0003-x
Egbueri JC (2018) Assessment of the quality of groundwaters proximal to dumpsites in Awka and Nnewi metropolises: a comparative approach. Int J Energy Water Res. https://doi.org/10.1007/s42108-018-0004-1
Mgbenu CN, Egbueri JC (2019) The hydrogeochemical signatures, quality indices and health risk assessment of water resources in Umunya district, southeast Nigeria. Appl Water Sci. https://doi.org/10.1007/s13201-019-0900-5
Deutsch WJ (1997) Groundwater geochemistry; fundamentals and applications to contamination. Lewis Publishers, New York
Ayotte JD, Gronberg JM, Apodaca LE (2011) Trace elements and radon in groundwater across the United States: US Geological Survey scientific investigations report 5059, 115p
Saba N, Umar R (2016) Hydrogeochemical assessment of Moradabad city, an important industrial town of Uttar Pradesh, India. Sustain Water Resour Manag 2:217–236
Egbueri JC (2019) Water quality appraisal of selected farm provinces using integrated hydrogeochemical, multivariate statistical, and microbiological technique. Model Earth Syst Environ. https://doi.org/10.1007/s40808-019-00585-z
Paul JH, Rose JB, Jiang S, Kellogg C, Shinn EA (1995) Occurrence of fecal indicator bacteria in surface waters and the subsurface aquifer in Key Largo, Florida. Appl Environ Microbiol 61(6):2235–2241
Ekenta OE, Okoro BU, Ezeabasili ACC (2015) Hydrogeological characteristics and groundwater quality analysis for selected boreholes in Ogbaru local government area, Anambra State, Nigeria. ASRJETS 14(2):198–210
Nfor BN, Olobaniyi SB, Ogala JE (2007) Extent and distribution of groundwater resources in parts of Anambra State, Southeastern Nigeria. J Appl Sci Environ Manag 11(2):215–221
Domenico PA, Schwartz FW (1990) Physical and chemical hydrogeology. Wiley, New York
APHA (2005) Standard methods for the examination of water and wastewater, 21st edn. American Public Health Association American Water Works Association/Water Environment Federation, Washington DC
Briancesco R (2005) Microbial indicators and fresh water quality assessment. Ann Ist Super Sanita 41(3):353–358
Douterelo I, Boxall JB, Deines P, Sekar R, Fish KE, Biggs CA (2014) Methodological approaches for studying the microbial ecology of drinking water distribution systems. Water Res 65:134–156
WHO (2011) Guidelines for drinking water quality, 3rd edn. World Health Organization, Geneva
NSDWQ (2007) Nigerian standard for drinking water quality. Nigeria Ind Stand 554:13–14
Pitt R, Clark S, Field R (1999) Groundwater contamination potential from storm water infiltration practices. Urban Water 1:217–236
Fetter CW (1990) Applied hydrogeology. Mirril Publ. Co., USA
WHO (2008) Guidelines for drinking-water quality, vol. 1, 3rd edn. Incorporating the first and second addenda. World Health Organization, Geneva
Todd DK (1980) Groundwater hydrology, 2nd edn. Wiley, New York
Rao NS (2006) Nitrate pollution and its distribution in the groundwater of Srikakulam district, Andhra Pradesh, India. Environ Geol 51(4):631–645
Adimalla N (2019) Spatial distribution, exposure, and potential health risk assessment from nitrate in drinking water from semi-arid region of South India. Hum Ecol Risk Assess: Int J. https://doi.org/10.1080/10807039.2018.1508329
Krishna-Kumar S, Bharani R, Magesh NS, Godson PS, Chandrasekar N (2014) Hydrogeochemistry and groundwater quality appraisal of part of south Chennai coastal aquifers, Tamil Nadu, India using WQI and fuzzy logic method. Appl Water Sci 4:341–350
Cushing EM, Kantrowitz IH, Taylor KR (1973) Water resources of the Delmarva Peninsula. US Geological Survey professional paper 822, Washington DC
Ahamed AJ, Loganathan K, Jayakumar R (2015) Hydrochemical characteristics and quality assessment of groundwater in Amaravathi river basin of Karur district, Tamil Nadu, South India. Sustain Water Resour Manag 1:273–291
Piper AM (1944) A graphical procedure in the geochemical interpretation of water analysis. Am Geophys Union Trans 25:914–928
Nemcic-Jurec J, Singh SK, Jazbec A, Gautam SK, Kovac I (2017) Hydrochemical investigations of groundwater quality for drinking and irrigational purposes: two case studies of Koprivnica-Krizevci County (Croatia) and district Allahabad (India). Sustain Water Resour Manag. https://doi.org/10.1007/s40899-017-0200-x
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Sci J 170:795–840
Kumar SK, Rammohan V, Sahayam JD, Jeevanandam J (2009) Assessment of groundwater quality and hydrogeochemistry of Manimuktha River basin, Tamil Nadu, India. Environ Monit Assess 159:341–351
Egbueri JC, Mgbenu CN, Chukwu CN (2019) Investigating the hydrogeochemical processes and quality of water resources in Ojoto and environs using integrated classical methods. Model Earth Syst Environ. https://doi.org/10.1007/s40808-019-00613-y
Adimalla N, Vasa SK, Li P (2018) Evaluation of groundwater quality, Peddavagu in Central Telangana (PCT), South India: an insight of controlling factors of fluoride enrichment. Model Earth Syst Environ 4(2):841–852. https://doi.org/10.1007/s40808-018-0443-z
Narsimha A, Sudarshan V (2017) Assessment of fluoride contamination in groundwater from Basara, Adilabad District, Telangana State, India. Appl Water Sci 7(6):2717–2725. https://doi.org/10.1007/s13201-016-0489-x
Narsimha A, Sudarshan V (2017) Contamination of fluoride in groundwater and its effect on human health: a case study in hard rock aquifers of Siddipet, Telangana State, India. Appl Water Sci 7(5):2501–2512. https://doi.org/10.1007/s13201-016-0441-0
Sakram G, Adimalla N (2018) Hydrogeochemical characterization and assessment of water suitability for drinking and irrigation in crystalline rocks of Mothkur region, Telangana State, South India. Appl Water Sci 8(5):143. https://doi.org/10.1007/s13201-018-0787-6
Anatole D (1996) Physical geology. D.C. Heath and Company, Lexington, p 628p
Datta PS, Tyagi SK (1996) Major ion chemistry of groundwater in delhi area: chemical weathering processes and groundwater flow regimes. J Geol Soc India 47:179–188
Meyback M (1987) Global chemical weathering of surficial rocks estimated from river dissolved loads. Am J Sci 287:401–428
Singh G (1987) Mine water quality deterioration due to acid mine drainage. Int J Mine Water 6:49–61
Al-Ruwaih FM, Ben-Essa SA (2004) Hydrogeological and hydrogeochemical study of the Al-Shagaya 460 Field-F, Kuwait. Bull Eng Geol Environ 63:57–70
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Egbueri, J.C. Evaluation and characterization of the groundwater quality and hydrogeochemistry of Ogbaru farming district in southeastern Nigeria. SN Appl. Sci. 1, 851 (2019). https://doi.org/10.1007/s42452-019-0853-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-019-0853-1