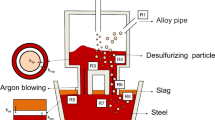
Abstract
A mathematical model for RH decarburization was developed on the basis of the principle of carbon and oxygen balance. In the simulation, a physical description of the different reaction sites associated with the different mechanisms was considered. Furthermore, the oxygen transfer between top slag and molten steel was also considered. The calculated results of the model were in good agreement with the operation data of the two different plants. The model was applied to analyze the effect of KTB starting time on the RH decarburization rate for ultra-low carbon steel production using slag reforming or LF refining. According to the results, the RH decarburization rate especially the inner site decarburization rate is controlled by the KTB starting time. In actual operation, late KTB starting time should be avoided.
Similar content being viewed by others
Abbreviations
- α:
-
Constant, α=4/3(Do/DC)0.5
- aFeO:
-
Activity of FeO
- aFeO(RS):
-
Activity of FeO calculated by regular model
- Ai:
-
Reaction surface, m2
- Ci:
-
Carbon content in interface, 10−6
- CE,i:
-
Equilibrium carbon content in interface, 10−6
- CL, CV:
-
Carbon content in molten steel in ladle and vessel, 10−6
- FeOS:
-
FeO content in slag related to mass of steel, mass%
- g:
-
Acceleration of gravity, m·s·2
- G:
-
Ar gas fowrate, L·min−1
- OE,i:
-
Equilibrium oxygen content in interface, 10−6
- OL, OV:
-
Oxygen content in molten steel in ladle and vessel, 10−6
- HCO:
-
Depth of CO formation region, m
- KC,i:
-
Transfer coeffcient of carbon, m·s−1
- KV:
-
Volumetric coeffcient for decarburization as CO bubbles, %·(Pa·s)−1
- KCO:
-
Equilibrium constant of interfacial reaction, Pa·%−2
- MC, MO:
-
Molecular mass of C and O, g·mol−1
- MFeO:
-
Molecular mass of FeO, g·mol−1
- nCO:
-
Amount of CO in a bubble, mol
- nCO,r:
-
Amount of CO absorbed by rising Ar bubbles, mol
- OLQ:
-
Equilibrium oxygen content in ladle, 10−6
- PCO,i:
-
Partial pressure in interface, Pa
- PV:
-
Vessel pressure, Pa
- Q:
-
Steel circulation rate, t·min−1
- ROSlag:
-
Oxygen transfer rate between top slag and molten steel, 10−6 s−1
- ROKTB:
-
Supply oxygen rate of KTB, 10−6 s−1
- t:
-
Time, s
- T:
-
Molten steel temperature, K
- TOL:
-
Reaction time constant for FeO reduction, s
- WL, WV:
-
Mass of molten steel in ladle and vessel, t
- WSlag:
-
Mass of top slag, kg
- XFeO:
-
Mole fraction of FeO
- ρ:
-
Density of steel, t·m−3
- γFeO:
-
Activity coeffcient of FeO
- τ:
-
Time required for Ar bubble away from the nozzle to the bath surface, s
- ΔCi:
-
Decarburization amount of Ar bubbles, bath surface and inner site, g·s−1
- ΔOi:
-
Deoxidation amount of Ar bubbles, bath surface and inner site, g·s−1
- i:
-
Decarburization site, i=Ar, Surface, Inner
References
M. Nadif, D. Brachet, in: 1989 Steelmaking Conference Proceedings, Chicago, 1989, pp. 227–233.
K. Yamaguchi, Y. Kishimoto, T. Sakuraya, T. Fujii, M. Aratani, H. Nishikawa, ISIJ Int. 32 (1992) 126–135.
K. Uemura, M. Takahashi, S. Koyama, Kobe Steel Eng. Rep. 41 (1991) No.4, 24–27.
S. Kitamura, M. Yano, K. Harashima, N. Tsutsumi, Tetsu-to-Hagané 80 (1994) No.3, 213–218.
M. Takahashi, H. Matsumoto, T. Saito, ISIJ Int. 35 (1995) 1452–1458.
T. Kitamura, K. Miyamoto, R. Tsujino, S. Mizoguchi, K. Kato, ISIJ Int. 36 (1996) 395–401.
H. Saint-Raymond, D. Huin, F. Stouvenot, Mater. Trans. JIM 41 (2000) No.1, 17–21.
J. H. Wei, N. W. Yu, Steel Res. Int. 73 (2002) No.4, 135–142.
Y. G. Park, K. W. Yi, ISIJ Int. 43 (2003) 1403–1409.
M. Y. Zhu, Y. L. Wu, C. W. Du, Z. Z. Huang, J. Iron Steel Res. Int. 12 (2005) No.2, 20–24.
J. M. Zhang, L. Liu, X. Y. Zhao, S. W. Lei, Q. P. Dong, ISIJ Int. 54 (2014) 1560–1568.
S. Y. Kitamura, H. Aoki, K. I. Miyamoto, H. Furuta, K. Yamashita, K. Yonezawa, ISIJ Int. 40 (2000) 455–459.
B. Kleimt, S. Kohle, H. J. Ponten, W. Matissik, D. Schewe, Ironmak. Steelmak. 20 (1993) 390–395.
B. Y. Shiro, ISIJ Int. 33 (1993) 2–11.
S. H. Kim, B. Song, Metall. Mater. Trans. B 33 (1999) 435–442.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Ph., Wu, Qj., Hu, Wh. et al. Mathematical simulation of behavior of carbon and oxygen in RH decarburization. J. Iron Steel Res. Int. 22 (Suppl 1), 63–67 (2015). https://doi.org/10.1016/S1006-706X(15)30140-0
Published:
Issue Date:
DOI: https://doi.org/10.1016/S1006-706X(15)30140-0