The regulation of energy homeostasis requires a specific detection of nutrients by specialized cells of the enteric area when absorbed by the digestive tract. The first actor of energy sensing is the intestinal epithelium where numerous endocrine cells are located. L-cells are the second most abundant population of endocrine cells in the human intestine, exceeded only by the population of enterochromaffin cells. A high abundance of L-cells is present in the colon (Moody, Reference Moody1980; Bryant et al. Reference Bryant, Bloom, Polak, Hobbs, Domschke, Domschke, Mitznegg, Ruppin and Demling1983; Sjolund et al. Reference Sjolund, Sanden, Hakanson and Sundler1983; Eissele et al. Reference Eissele, Goke, Willemer, Harthus, Vermeer, Arnold and Goke1992). L-cells from the intestine are thought to arise from pluripotent stem cells in the crypts that also give rise to enterocytes, goblet cells and Paneth cells (Fujita et al. Reference Fujita, Cheung and Kieffer2004). Stem cells located in the crypts differentiate into the four cell types present in the epithelium. Notch proteins mediate cell fate decisions and patterning by regulating expression of basic-helix–loop–helix (bHLH) transcription factors that control terminal differentiation (Schonhoff et al. Reference Schonhoff, Giel-Moloney and Leiter2004). The sequential appearance of Math1, neurogenin 3 (NGN3) and BETA2/NeuroD (NeuroD) may represent distinct stages in the differentiation of enteroendocrine cells. NGN3 and NeuroD specifically drive cells into enteroendocrine cell types such as L-cells. The nature of positional cues that direct the distribution of each cell type has not been characterized yet (Fujita et al. Reference Fujita, Cheung and Kieffer2004).
Glucagon-like peptide-1 (GLP-1) is a key hormone released from enteroendocrine L-cells in response to nutrient ingestion (Orskov et al. Reference Orskov, Holst, Knuhtsen, Baldissera, Poulsen and Nielsen1986). GLP-1 is produced by a tissue-specific post-translational processing of its precursor proglucagon peptide by prohormone convertase 1 enzymes (Dhanvantari et al. Reference Dhanvantari, Seidah and Brubaker1996). It promotes insulin secretion and β-cell proliferation in the pancreas, controls glycogen synthesis in muscle cells and promotes satiety (Brubaker & Drucker, Reference Brubaker and Drucker2004; Holst, Reference Holst2004; Knauf et al. Reference Knauf, Cani and Perrin2005). These actions render GLP-1 highly attractive as a therapeutic agent, but a rapid enzymatic degradation of the molecule by dipeptidyl peptidase IV makes it unsuitable for injection (Deacon et al. Reference Deacon, Knudsen, Madsen, Wiberg, Jacobsen and Holst1998; Meier et al. Reference Meier, Nauck, Kranz, Holst, Deacon, Gaeckler, Schmidt and Gallwitz2004). Therefore, two pharmacological strategies are being pursued: the development of dipeptidyl peptidase IV-resistant analogues of GLP-1 and the development of dipeptidyl peptidase IV inhibitors. A way to promote endogenous GLP-1 secretion or stability would be useful in that context.
We and others have shown that non-digestible carbohydrates which are largely fermented in the colon, such as oligofructose (OFS), when added in the diet, improve glucose tolerance, insulin secretion and lower food intake in animals and in man. These effects are often associated with a higher plasma GLP-1 content (Yamashita et al. Reference Yamashita, Itakura and Kawai1984; Piche et al. Reference Piche, des Varannes, Sacher-Huvelin, Holst, Cuber and Galmiche2003; Cani et al. Reference Cani, Dewever and Delzenne2004, Reference Cani, Daubioul, Reusens, Remacle, Catillon and Delzenne2005a, Reference Cani, Neyrinck, Maton and Delzenneb, Reference Cani, Joly, Horsmans and Delzenne2006a). We have recently shown that the effects of OFS were abolished when it was given in the diet of GLP-1 Receptor knock-out mice, as well as in mice chronically treated with GLP-1 Receptor antagonist (exendin 9-39; Cani et al. Reference Cani, Knauf, Iglesias, Drucker, Delzenne and Burcelin2006b). The mechanisms underlying the relation between non-digestible carbohydrate ingestion and GLP-1 production remain unknown. Some authors have proposed that the fermentation of non-digestible carbohydrate into SCFA allows those metabolites to promote proglucagon expression in intestine cells (Reimer & McBurney, Reference Reimer and McBurney1996; Reimer et al. Reference Reimer, Thomson, Rajotte, Basu, Ooraikul and McBurney1997; Massimino et al. Reference Massimino, McBurney, Field, Thomson, Keelan, Hayek and Sunvold1998). In the present study, we test the hypothesis that non-digestible carbohydrate could also target the enteroendocrine L-cell differentiation pathway.
Materials and methods
Animals and diets
Male Wistar rats (ten rats per group) weighing 145–160 g (Harlan, Horst, The Netherlands) were housed in individual cages in a temperature- and humidity-controlled room with a 12 h light–dark cycle. After an acclimation period of 5 d before the experiment, control rats were fed a powdered A04 standard diet (A04; UAR, Villemoisson-sur-Orge, France), whereas OFS-treated rats received a diet prepared by mixing 90 g A04 standard diet with 10 g corresponding fructan: Raftilose P95 (Orafti, Tienen, Belgium; Cani et al. Reference Cani, Dewever and Delzenne2004). The A04 standard diet contained the following (g/100 g dry diet): 19·3 protein (consisting of equivalent mix of soya and fish proteins); 70·4 total carbohydrates obtained from maize, wheat, barley and bran (including 38 starch, 3 saccharose, 5 cellulose and 8 non-digestible carbohydrates); 3 lipids; 6 mineral mixture and 1·3 vitamins. Food intake, taking into account spillover, was assessed three times per week. The mean daily energy intake (kJ/d) was calculated as follows: food intake (g) × energy value of diet (kJ/g). The energy value for the control diet was 13·86 kJ/g and for the OFS diet it was 13·08 kJ/g.
Rats were killed after 4 weeks of treatment. All experiments were approved by the local committee and the housing conditions were as specified by the Belgian Law of 14 November 1993 on the protection of laboratory animals (agreement no. LA 1230314).
Chemicals
Raftilose P95 is a mixture of glucosyl-(fructosyl)n-fructose and (fructosyl)m-fructose but with an average degree of polymerisation of 4·5. Other chemicals used in the present study, of the purest grade available, were purchased from Sigma (St Louis, MO, USA) and Merck (Darmstadt, Germany).
Blood samples
On day 28, food was withheld and 8 h later rats were anaesthetized by intraperitoneal injection of sodium pentobarbital solution (using 60 mg Nembutal®/kg body weight; Sanofi Santé Animale Benelux, Brussels, Belgium). Portal vein blood samples were collected in EDTA tubes (Sarstedt, Nümbrecht, Germany) containing dipeptidyl peptidase IV inhibitor (Linco Research, St Charles, MO, USA); after centrifugation, plasma was stored at − 80°C. GLP-1 (7–36) amide was measured using an ELISA Kit, specific for GLP-1 (7–36) amide without cross-reactivity towards GLP-1 (9–36) amide, glucagon-like peptide-2 and glucagon (GLP-1 Active ELISA Kit; Linco Research).
Tissue samples
Colon was immediately excised, flushed with ice-cold saline buffer and divided into 2 cm segments taken just after the caecal junction, in the middle of the colon and just before the rectum, corresponding to proximal, medial and distal colon, respectively. Segments were immersed in liquid nitrogen, and stored at − 80°C, for further analysis, or fixed overnight in 4 % formaldehyde in PBS and routinely paraffin-embedded. Full and empty caecum, and adipose tissues (epidydimal, inguinal, visceral and brow adipose tissue), were weighed.
Intestinal peptide extractions
Extraction of GLP-1 (7–36) amide from intestinal segments (caecum and colon) was carried out with ethanol–acid (100 % ethanol–sterile water–12 m-HCl, 74:25:1) solution using 5 ml/g tissue. Samples were homogenized at 24 000 rpm and placed for 24 h at 4°C. Homogenates were centrifuged (20 min at 2000 g), and supernatant was decanted and diluted 200- and 500-fold in saline for caecum and colon, respectively. Concentrations of intestinal GLP-1 (7–36) amide were measured as previously described for blood samples.
Quantitative RT–PCR
Total RNA was isolated from intestine with TriPure Isolation Reagent (Roche, Basel, Switzerland) and single-strand cDNA was synthesized from 1 μg total RNA by using oligo dT (Reverse Transcription Kit; Promega, Madison, WI, USA). Quantitative PCR was carried out with first-strand cDNA with primers for proglucagon sense, 5′-gtaatgctggtacaaggcag-3′ and 5′-ttgatgaagtctctggtggca-3′; for NGN3 sense, 5′- aagagcgagttggcactgagc-3′; antisense, 5′-aagctgtggtccgctatgcg-3′; for NeuroD sense, 5′-cttggccaagaactacatctgg-3′; antisense, 5′-cgtgtttgaaagagaagttgcc-3′; and as internal control RPL-19 sense, 5′-gaaggtcaaagggaatgtgttca-3′; antisense, 5′-ccttgtctgccttcagcttgt-3′ on an ABI Prism 5700HT sequence detection system, with SYBR green reagents (Applied Biosystems, Foster City, CA, USA), the threshold cycles (Ct) were measured in separate tubes and in duplicate, and data were analysed according to the 2− ΔCt method. The identity and purity of the amplified product was checked through the analysis of the melting curve carried out at the end of amplification.
Immunohistology
Paraffin sections (3 μm thickness) of proximal colon were immunostained by a rabbit anti-GLP-1 antibody (GLP-1 antibody is directed against the N-terminal part of the GLP-1, leading to the detection of active form of GLP-1 (7–36 amide and 7–37); 1:4000, overnight incubation at 4°C; Phoenix Pharmaceuticals, Mountain View, CA, USA) combined with a classical streptavidin–biotin–peroxidase detection system. Finally, the peroxidase activity was revealed by immersion in a solution of 3,3′-diaminobenzidine hydrochloride (0·05 % 3,3′-diaminobenzidine, brown deposit; Fluka, Buchs, Switzerland). Sections were counter-stained by Hemalun of Mayer (10 s), dehydrated and coverslipped with DPX mounting medium.
The number of immunopositive cells in the intestinal segments was counted in two non-serial immunostained sections, using a light microscope (final magnification 200 × ), by an investigator blind to the experimental group. On the same material, mucosa area was manually delineated by the investigator and measured by a semiautomatic image analyser (Videoplan Kontron, Munich, Germany). Results are expressed as the number of L-cells per mucosal area (number/mm2).
Statistical analysis
Results are expressed as means and their standard errors. Statistical differences between groups were evaluated by Student's t test using GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego, CA, USA; www.graphpad.com). The level of significance was set at P < 0·05.
Results
Modulation of food intake, body weight and adiposity by oligofructose
Compared to the control diet, OFS feeding significantly reduced food intake, energy intake and body weight gain (Fig. 1(A–D)). This was associated with lower epidydimal, inguinal and visceral adipose tissue, by about 30, 43 and 39 %, respectively. There was no effect on brown adipose tissue weight (Fig. 1(B)).
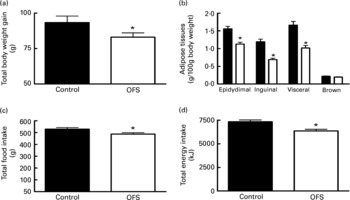
Fig. 1 The effect of oligofructose (OFS) on body weight gain (g) (A), adipose tissues (g/100 g body weight) (B), total food intake (g) (C) and cumulative energy intake (kJ) (D), of rats fed a control diet (■) or a diet supplemented with OFS (□).Values are means with their standard errors depicted by vertical bars (n 10). Mean values were significantly different from those of the control group: *P < 0·05.
Markers of fermentation
Macroscopic analysis of the organs revealed caecum enlargement in OFS-fed rats. The total caecum weight was significantly higher in OFS-fed than in control rats (control, 4·37 (sem0·32) g; OFS, 10·75 (sem 0·8) g; P < 0·05). The caecal tissue weight was significantly higher in OFS-fed than in control rats (control, 1·07 (sem 0·06) g; OFS, 1·8 (sem 0·06) g; P < 0·05).
Modulation of glucagon-like peptide-1 production by oligofructose
OFS feeding doubled portal GLP-1 plasma levels (Fig. 2(A)). We did not observe any modulation of proglucagon mRNA and GLP-1 content in the ileum, caecum and distal colon (data not shown). Therefore, we decided to focus on the proximal colon. Fig. 2 shows a similar increase (two-fold, versus control) of GLP-1 concentration and proglucagon mRNA in the proximal colon.

Fig. 2 The effect of oligofructose (OFS) on portal glucagon-like peptide-1 (GLP-1; 7–36) amide concentration (pmol/l) (A), proximal colon GLP-1 concentrations (pmol/g) (B) and proglucagon mRNA contents (C) in the proximal colon of rats fed a control diet (■) or a diet supplemented with OFS (□). Values are means with their standard errors depicted by vertical bars (n 10). Mean values were significantly different from those of the control group: *P < 0·05.
The number of GLP-1-positive L-cells in the proximal colon was also almost doubled following OFS treatment (Fig. 3(A)).

Fig. 3 The effect of oligofructose (OFS) on number of L-cells per mm2 (A), neurogenin 3 (NGN3) mRNA content (B) and NeuroD mRNA content (C) in the proximal colon of rats fed a control diet (■) or a diet supplemented with OFS (□). Values are means with their standard errors depicted by vertical bars (n 10). Mean values were significantly different from those of the control group: *P < 0·05.
The histological analysis of colon segments showed no modifications of crypt height and numbers, and no sign of epithelial cell proliferation or apoptosis.
NGN3 is a key factor known to initiate endocrine differentiation; it activates the expression of BETA 2/NeuroD, which coordinates terminal differentiation (Fujita et al. Reference Fujita, Cheung and Kieffer2004). We measured these two factors and found, in the proximal colon of OFS-fed animals, a significant increase of NGN3 and NeuroD expression, by about 2·7- and 1·4-fold, respectively (Fig. 3(B, C)). The present results suggest that OFS promotes the differentiation of cell precursors (stem cells) into mature L-cells, by the promoting expression of key differentiation factors.
Discussion
The present study confirms that non-digestible/fermentable carbohydrates, namely OFS, significantly increase proglucagon mRNA and GLP-1 peptide contents in the proximal colon without any modulation in the other intestinal segments (Cani et al. Reference Cani, Dewever and Delzenne2004, Reference Cani, Daubioul, Reusens, Remacle, Catillon and Delzenne2005a, Reference Cani, Neyrinck, Maton and Delzenneb). OFS decreases food and energy intake, body weight gain and fat mass accumulation. This is associated with a significant increase of GLP-1 in the portal plasma vein and in the proximal colon tissue. We demonstrate for the first time that targeting the proximal colon by a non-digestible/fermentable compound promotes the endogenous GLP-1 content and that this effect is related to the increase in number of endocrine L-cells.
L-cells – as other intestinal cells – come from common stem cells. How stem cells are determined to differentiate into enteroendocrine cells is not completely understood. Controversies have been persisting for many years, questioning whether each endocrine cell type differentiates from its own precursor, or whether all enteroendocrine cells segregate from a common progenitor cell. The sequential appearance of Math1, NGN3 and NeuroD may represent distinct stages in the differentiation of enteroendocrine cells (Schonhoff et al. Reference Schonhoff, Giel-Moloney and Leiter2004). The nature of positional cues that direct the distribution of each cell type have not been characterized yet (Fujita et al. Reference Fujita, Cheung and Kieffer2004). NGN3 is considered to be a pro-endocrine factor, since it is not identified in differentiated enteroendocrine cells, suggesting that this factor is transiently expressed and switched off prior to terminal differentiation (Schwitzgebel et al. Reference Schwitzgebel, Scheel, Conners, Kalamaras, Lee, Anderson, Sussel, Johnson and German2000). A recent report demonstrates a direct relationship between the number of enteroendocrine cells and the expression of differentiation factors (Shroyer et al. Reference Shroyer, Wallis, Venken, Bellen and Zoghbi2005). Here, we observe that the doubling of the number of L-cells in the proximal colon upon OFS feeding is associated with a significant increase in NGN3 and NeuroD mRNA contents. The present results suggest a promotion of L-cell differentiation in the proximal colon by OFS. The only way to clearly show that NGN3 and NeuroD are key factors to explain the higher differentiation under OFS would be the use of specific tools to block those differentiation factors, the corresponding knock-out animals die post-natally (Gradwohl et al. Reference Gradwohl, Dierich, LeMeur and Guillemot2000). The putative mechanism whereby OFS induce L-cell differentiation remains unknown. We may propose that OFS fermentation end-products such as SCFA (i.e. acetate, propionate and butyrate) are involved in such effects (Roberfroid & Delzenne, Reference Roberfroid and Delzenne1998). Butyrate has been proposed as key regulator of intestinal cell differentiation in vitro, and appears as the best candidate to explain the putative modulation of colonic cell metabolism by modulating gene expression (Mariadason et al. Reference Mariadason, Barkla and Gibson1997, Reference Mariadason, Rickard, Barkla, Augenlicht and Gibson2000; Tappenden et al. Reference Tappenden, Drozdowski, Thomson and McBurney1998; Hodin, Reference Hodin2000). In fact, several studies have shown that butyrate is able to increase proglucagon gene expression in immortalised L-cells in vitro; the infusion of butyrate in the colon also increases proglucagon and GLP-1 contents (Dumoulin et al. Reference Dumoulin, Dakka, Plaisancie, Chayvialle and Cuber1995; Pratt et al. Reference Pratt, Tappenden, McBurney and Field1996). Moreover, the demonstration that the SCFA receptor GPR43 is expressed on L-cells puts forward that SCFA may be involved in the release of GLP-1 (Karaki et al. Reference Karaki, Mitsui, Hayashi, Kato, Sugiya, Iwanaga, Furness and Kuwahara2006). A previous study reports that in OFS-treated rats, intestinal butyrate concentration is doubled (Le Blay et al. Reference Le Blay, Michel, Blottiere and Cherbut1999). Moreover, the profile of SCFA – the relative proportion of acetate, propionate and butyrate – in the colonic content differs following the degree of polymerisation of non-digestible oligosaccharides ingested by rats (Nyman, Reference Nyman2002). The SCFA content is not similar in the caecal and colonic content of rats treated with OFS. The butyrate proportion was higher both in the caecum and proximal colon of OFS-fed animals than in control animals, while a higher butyrate level was found in the proximal colon than in the caecum. The butyrogenic properties of OFS have been recently confirmed (Nilsson & Nyman, Reference Nilsson and Nyman2005).
Therefore, if butyrate is produced in a sufficient amount, it could contribute to increased GLP-1 production by both mechanisms: L-cell differentiation and/or increased proglucagon expression.
In conclusion, we confirm that non-digestible carbohydrates such as OFS increase endogenous GLP-1 production and portal plasma levels, a phenomenon contributing to control of body weight and adiposity. We demonstrate for the first time a relation between GLP-1 content, number of L-cells, NGN3 and NeuroD mRNA level in the colon: we thus propose that targeting the proximal colon with non-digestible dietary compound could increase L-cell differentiation by mechanisms linked to NGN3 and NeuroD up-regulation. The present results fit with the idea that events occurring in the colon (fermentation; modulation of gut microbiota) exert a key influence on the overall host metabolism (Backhed et al. Reference Backhed, Ding, Wang, Hooper, Koh, Nagy, Semenkovich and Gordon2004; Dumas et al. Reference Dumas, Barton and Toye2006). While the doses used in animal studies are not directly transposable to human nutrition, several reports suggest that the ingestion of fructo-oligosaccharides at the dose of 10–20 g/d promotes physiological effects. A recent report demonstrates that OFS feeding (20 g/d) significantly increases plasma GLP-1 after a mixed meal (Piche et al. Reference Piche, des Varannes, Sacher-Huvelin, Holst, Cuber and Galmiche2003). We have recently shown, in healthy human subjects, that feeding 16 g/d OFS promotes satiety following breakfast and dinner, and reduces hunger and prospective food consumption after dinner. This was accompanied by a significant 10 % lower total energy intake (Cani et al. Reference Cani, Joly, Horsmans and Delzenne2006a). Moreover, Archer et al. (Reference Archer, Johnson, Devereux and Baxter2004) have demonstrated that fermentable fructo-oligosaccharides, added to food as a fat replacer, were able to lower energy intake during a test day. The role of fermentable dietary fibres in the management of appetite in healthy human subjects has been recently confirmed (Whelan et al. Reference Whelan, Efthymiou, Judd, Preedy and Taylor2006). It also gives a scientific rationale to promote the ingestion of fermentable dietary fibres – including oligosaccharides – to help control obesity and associated metabolic disorders.
Acknowledgements
P. D. C. is a Postdoctoral Researcher from the Fonds National pour la Recherche Scientifique (FNRS), Belgium. This work was supported by a FSR grant from the Université catholique de Louvain and FNRS grant 1.5.231.06. The authors thank Prof. I. Leclercq for providing access to q-PCR techniques and helpful criticisms. We also thank N. Maton for excellent technical assistance.







