Enteric fermentation in livestock accounts for 19 % of anthropogenic sources of methane, a potent greenhouse gas(Reference Johnson and Johnson1), for which rumen fermentation is the largest source of methane production. In rumen fermentation, several pathways involving both hydrogen-producing and -consuming steps are involved in the conversion of feedstuffs into various fermentation end products such as SCFA(Reference Ungerfeld, Kohn, Sejrsen, Hvelplund and Nielsen2, Reference Moss, Jouany and Newbold3). Although metabolic hydrogen in the rumen is incorporated in fermentation end products by bacteria, methanogenic archaea (methanogens) consume the greater majority of metabolic hydrogen to obtain energy for their metabolism and finally release methane, which accounts for 2–12 % loss of the metabolic energy from feed(Reference Johnson and Johnson1, Reference Moss, Jouany and Newbold3, Reference Morgavi, Forano and Martin4). Therefore, management of metabolic hydrogen and methane production in the rumen is an important factor to be considered, when developing strategies to reduce greenhouse gas emissions and improve efficiency of energy utilisation from feed.
It is known that many chemical agents such as ionophores (e.g. monensin), unsaturated fatty acids, sulphate, nitrate, fumarate and halogenated methane analogues (e.g. bromochloromethane (BCM)) are able to reduce methane production from ruminants(Reference Johnson and Johnson1, Reference Morgavi, Forano and Martin4–Reference Itabashi, Takahasi and Young6). BCM is one of the most effective inhibitors and apparently reduces methane production by interfering with the cobamide-dependent methyl transferase step of methanogenesis(Reference Wood, Kennedy and Wolfe7, Reference Chalupa8). BCM complexed in cyclodextrin (CD; BCM-CD) results in the sustained inhibition of methane production when fed to ruminants(Reference May, Payne and Stewart9–Reference Tomkins and Hunter11). Moreover, an in vitro continuous fermentation system simulating rumen fermentation demonstrated that BCM significantly reduced methane production (85–90 %) and eliminated most methanogens, whereas there was no effect on total SCFA production, true degradability of feed and efficiency of microbial protein synthesis(Reference Goel, Makkar and Becker12). Although H2 gas was not measured in the in vitro study, it is predicted that H2 gas would accumulate in the rumen when methanogenesis is strongly inhibited by suppression of growth of ruminal methanogens(Reference Janssen13).
The aims of the present study were to analyse the effect of BCM-CD on in vivo methane production, metabolic hydrogen flux and subsequent responses in SCFA production and rumen microbial community structure of goats. Varying dose levels of BCM-CD were administered to the rumen of goats fed a 1·1 × maintenance diet of roughage and concentrate (1:1) in open-circuit respiration chambers. It was hypothesised that (1) the rumen microbiota would adapt to inhibition of methanogenesis and shift fermentation to reductive processes that would consume more reducing equivalents, but (2) excessive H2 gas would still accumulate and impair fibre digestion.
Materials and methods
Animals and experimental design
The Shiba goat, which is an inbred strain of Japanese miniature goat, has been established for experimental use(Reference Sakurai, Ohkura and Matsuyama14) and was used in the present study. Three fistulated Japanese native (Shiba) goats (Capra aegagrus hircus, female) 35·7 (sem 4·85) kg were fed a 1·1 × maintenance diet of 50 % of Timothy hay (Phleum pratense, g/kg; organic matter 949, neutral-detergent fibre 735, crude protein 49 and ash 51) and 50 % of concentrate (designed by National Institute of Livestock and Grassland Science: maize powder, 30 %; barley powder, 25 %; wheat bran, 9 %; defatted rice bran, 7 %; soybean meal, 11·7 %; molasses, 3·5 %; alfalfa meal, 7 %; beet pulp, 5 %; calcium carbonate, 1·2 %; NaCl, 0·5 %; vitamin premixed, 0·1 %; g/kg: organic matter, 937; neutral-detergent fibre, 243; crude protein, 185; ash 63), with which CD (0·46 g/100 kg live weight (LW)) was supplemented (376·5 (sem 39·0) g each) for an adaption period of 14 d before gas measurements and sampling. A sampling period of 8 d followed and served as the control collection period for all animals. Within the sampling period, goats were placed into the respiration chambers for a period of 3 d for analysis of rumen gas production. Animals were then adapted to a low dose of BCM-CD (0·5 g/100 kg LW), which was premixed in the maintenance diet without CD, for 8 d before sampling in the respiration chambers for 3 d. Doses were then increased to a mid dose (2 g/100 kg LW) and after 8 d of adaption, there was further 3 d of sampling. A final high dose (5 g/100 kg LW) of BCM-CD was administered and a similar adoption and sampling regime was followed as before, with gas analysis performed in the respiration chambers. The BCM-CD containing 8 % BCM was prepared by Ensuiko Sugar Refining Company Limited (Yokohama, Japan). Rumen samples were collected at the end of each respiration-sampling period (3 d) for analysis of fermentation end products and microbial ecology. Digestibility trials and measurement of methane production in whole animal, open-circuit, indirect respiration chambers equipped with gas analysers for oxygen, carbon dioxide and methane were performed according to the techniques and procedures described by Bhatta et al. (Reference Bhatta, Enishi and Takusari15). Briefly, methane concentration was accurately measured by sampling gas from the outlet of a chamber and actual production calculated from outflow rates of gas. H2 concentrations in a chamber were measured by a H2 monitor (Custom-made device; TAIYO Instruments Inc., Osaka, Japan), which was installed in each chamber, but actual production rate was not measured because H2 gas in each chamber exceeded the limit of detection (>65 parts per million) when methanogenesis was inhibited. The animal experiments were carried out in accordance with a protocol approved by the Guide for the Care and Use of Experimental Animals (Animal Care Committee, National Institute of Livestock and Grassland Science).
Organic acid sampling and analysis
For the detection of SCFA, a 1-ml sample of rumen fluid was taken just before the morning feed at various time points for analysis. After samples were centrifuged (12 000 g, 10 min), 600 μl of sample was combined with 150 μl of meta-phosphoric/internal standard solution (20 % meta-phosphoric acid/0·24 % 4-methyl valeric acid). Analysis of organic acids was performed using a Shimadzu GC-17A GC on a packed glass column (length 2 m; outer diameter 0·6 mm; inner diameter 0.2 mm) containing 10 % FFAPTM (free fatty acid phase)/1 % H3PO4 on Chromasorb WAW 100/120 mesh. The C2–C5 acids were separated over 16 min using nitrogen as a carrier at 12 ml/min. Peaks were detected by a flame ionisation detector(Reference Playne16). Lactate concentrations were determined from the supernatants of the centrifuged samples with the d-lactate and l-lactate assay kit (K-DLATE; Megazyme International Ireland Limited, Wicklow, Ireland) according to the manufacturer's instructions.
Calculation of hydrogen balance
The estimated recovery of metabolic hydrogen in the form of reduced protons (H) can be calculated from 2H utilised (2HU) and 2H produced (2HP) in rumen fermentation. The 2HP as a fermentation intermediate and 2H utilised in SCFA (2HUS) and methane were estimated from the amount and molar proportions of acetate (C2), propionate (C3), butyrate (C4), iso-valerate (Ci5) and valerate (C5) and from molar concentration of methane, as described in the following(Reference Goel, Makkar and Becker12, Reference Demeyer and Jouany17) equations:
As administration of BCM induced a marked increase in H2 gas production, which reached unmeasurable levels (>65 parts per million, Fig. 1), it was predicted that 2HU comprised 2HUS, 2H recovery in methane (2HUM) and 2H recovery in H2 (2HUH). Therefore, 2HU was calculated as
because the recovery rate of metabolic hydrogen, which is 2HU/2HP, is estimated as 0·9(Reference Moss, Jouany and Newbold3, Reference Demeyer and Jouany17, Reference Faichney, Graham and Walker18); however, in the controls, there was minimal H2 production as methane formation was not inhibited, and thus 2HU was calculated according to Goel et al. (Reference Goel, Makkar and Becker12) as:
Therefore, the equation used to calculate methane production in controls (Mcont) was Mcont = (2HU − 2HUS)/4 = ((0·9 × 2HP) − 2HUS)/4. Methane production at the low, mid and high doses of BCM (Mbcm; mmol/l) was calculated by using methane production in controls and methane reduction rates (MR; %), which were calculated from methane production in respiration chambers (Mrc; mol/head per d), as Mbcm = Mcont × (100 − MR)/100. H2 production at each dose of BCM (Hbcm) was calculated as a modification of equations 1 and 2:

Fig. 1 Hydrogen levels (parts per million (ppm)) detected within chambers for control (![]() ) and high dose of bromochloromethane (
) and high dose of bromochloromethane (![]() ). The feed was provided at time 0. Regions in a state of plateau on light-grey lines meant undetectable level of hydrogen (>65 ppm).
). The feed was provided at time 0. Regions in a state of plateau on light-grey lines meant undetectable level of hydrogen (>65 ppm).
The amount of H2 production in respiration chambers (Hrc) was estimated in mol as Hrc (mol/head per d) = Mrc (mol/head per d) × Hbcm/Mbcm. 2HUH was estimated as: 2HUH (%) = (Hbcm/2HP) × 100.
The relationship between measured methane production in chambers and (C2+C4)/C3 ratio were plotted on a chart according to the method reported by Moss et al. (Reference Moss, Jouany and Newbold3), in order to obtain an approximate curve.
Monitoring microbial populations
Quantitative PCR (qPCR) for monitoring three cellulolytic rumen bacterial species, F. succinogenes, R. albus and R. flavefaciens, anaerobic rumen fungi and methanogen populations were performed using published primers and assay conditions(Reference Denman and McSweeney19–Reference Kang, Denman and Morrison21). The qPCR was performed using a DNA template, total genomic DNA isolated from a 1-ml sample of rumen fluid using the FastDNA Kit and FastPrep Instrument (MP Biomedicals, Cleveland, OH, USA) in four technical replicates. Changes in targeted populations were calculated using a relative quantification calculation and the 2− ΔΔCt method, with the control period used as the calibrator and total bacterial(Reference Denman and McSweeney19) Ct (cycle threshold) values used as the reference value(Reference Livak and Schmittgen22). Estimation of abundance of target populations to indicate their contribution to the sample was also calculated using standard curves for each target gene generated from cloned PCR products. Protozoal populations were identified and counted microscopically after fixing and staining with methyl green–formalin–saline solution described by Ogimoto & Imai(Reference Ogimoto and Imai23). Denaturing gradient gel electrophoresis (DGGE) of 16S rRNA gene (recombinant DNA (rDNA)) PCR products was used to profile the diversity of rumen bacteria and was accomplished according to the method reported by Shinkai et al. (Reference Shinkai, Ueki and Kobayashi24) using 8 % (v/v) polyacrylamide DGGE gel with 35–65 % denaturant gradient. The PCR product, which was amplified with primers 1 and 2(Reference Muyzer, de Waal and Uitterlinden25) from the DNA excised from a DGGE gel, was inserted into a cloning vector, pCR2.1 (Invitrogen Corporation, Carlsbad, CA, USA). The sequence of cloned DNA fragment was determined and compared to the 16S rDNA sequences in the GenBank database using the DDBJ BLAST program (http://www.ddbj.nig.ac.jp/Welcome-e.html) and the Seq match program supported by Ribosomal database project (http://rdp.cme.msu.edu/).
Primer design and monitoring of Prevotella groups
Sequencing products from DGGE bands representing Prevotella sp. were aligned to 16S rRNA sequences in the latest greengenes ARB database(Reference DeSantis, Hugenholtz and Larsen26). Primers to be used in a qPCR assay to target these groups were designed using the most similar full-length sequence. Primers for Prevotella group 1 were designed from rumen clone F24-B12 (GenBank accession number, AB185591; prev_3_F, 5′-TGACGTCCTTCGGGACCG-3′; prev_3_R, 5′-TCCCGCCTTCTGTATGCC-3′) producing a 243 bp product from 1019 to 1262 (Escherichia coli numbering). Primers for Prevotella group 7 were designed from an uncultured rumen bacterium clone L102RC-4-F10 (GenBank accession no. HQ399809; prev_7_F, 5′-GTAGAGGATAGCCCGGCG-3′; prev_7_R, 5′-CTGATCGACGGCTTGG TG-3′) producing a 147 bp product from 141 to 287 (E. coli numbering). Primers were then compared with sequences available at the National Center for Biotechnology Information via a BLAST search to ascertain primer specificity(Reference Altschul, Gish and Miller27) and against the Ribosomal database project II and ARB databases using the probe match analysis function(Reference Cole, Chai and Marsh28, Reference Ludwig, Strunk and Westram29).
qPCR assays were performed on an ABI PRISM® 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA, USA). Assays were set up using the Platinum SYBR Green qPCR SuperMix-UDG (Invitrogen). Optimisation of assay conditions was performed for primer, template DNA and MgCl2 concentrations. An optimal primer concentration of 300 nm, a final MgCl2 concentration of 3 mm and DNA template concentration of between 1 and 100 ng were used for each assay under the following cycle conditions: one cycle of 50°C for 2 min and 95°C for 2 min for initial denaturation, forty cycles at 95°C for 15 s and 56°C for 1 min for primer annealing and product elongation. Fluorescence detection was performed at the end of each denaturation and extension step. Amplicon specificity was performed via dissociation curve analysis of PCR end products by raising the temperature at a rate of 1°C/30 s from 60 to 95°C. Total microbial rumen DNA from sampling time points was diluted in the ratio 1:10 before use in quantitative real-time PCR.
Statistical analyses
Experimental data, digestibility, gas, methane and SCFA were subjected to ANOVA using the Excel 2003 software (Microsoft Corporation, Redmond, WA, USA) with the add-in software Statcel2 (OMS Publishing, Inc., Saitama, Japan). Regression analyses, SCFA to methane and MR to SCFA, methane or H2, were carried out by simple regression analysis. Statistical analyses of the effect of BCM treatment on microbial populations were carried out by ANOVA, with differences determined by the method of least significant difference at the 5 % (P < 0·05) and 1 % (P < 0·01) levels using Statistica 6.0 software (StatSoft, Inc., Tulsa, OK, USA).
Results
Digestibility
All feed offered (1·1 × maintenance diet) was consumed by each goat and digestibility data were analysed for the control and highest dose of BCM. BCM did not exhibit a negative effect on digestibility compared to the control period (Table 1).
Table 1 Feed digestibility in goats
(Mean values with their standard errors, n 3 animals)
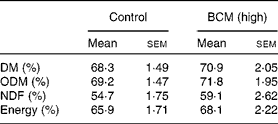
BCM, bromochloromethane; ODM, organic DM; NDF, neutral-detergent fibre.
Methane and hydrogen gas production
Daily quantities of methane produced from animals were reduced with respect to the dose level of BCM (Table 2). Significant decreases in methane production were evident at the mid and high doses, with a 71·3 and 91 % decrease, respectively, compared to the control. Real-time monitoring of the H2 gas showed that, in the control period, levels in expired gases were negligible (Fig. 1). When the highest dose of BCM was administered, the concentration of H2 rapidly accumulated in the chamber (above measurable levels>65 parts per million) and only decreased in concentration in the latter stages of monitoring each day, in relation to feeding (Fig. 1). The estimated H2 production in respiration chambers is shown in Table 2. Zero H2 production during the control period was used when estimating H2 production at various levels of BCM. A low level of H2 production (544·6 mmol/head per d) was estimated for the low BCM dose (5 % MR) compared with H2 production, which significantly increased by 6·4-fold at the high BCM dose (91 % MR).
Table 2 Measured methane and estimated hydrogen gas production levels in goats at various levels of bromochloromethane (BCM)
(Mean values with their standard errors, n 3 animals)
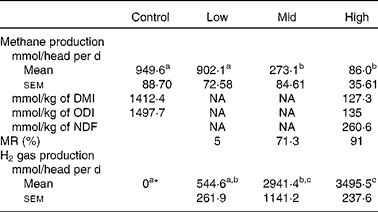
DMI, DM intake; NA, data not available; ODI, organic DM intake; NDF, neutral-detergent fibre; MR, methane reduction on a head basis.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* No hydrogen production is presupposed at control. Hydrogen levels at the low, mid and high doses of BCM were stoichiometrically estimated from methane production, MR and SCFA concentrations (see text).
Organic acid production
The total SCFA content ranged from 47·5 to 55·3 mmol/l, with no significant difference between treatments. Although concentrations of propionate and iso-valerate were significantly increased in the high dose (P < 0·05), there was no significant change in those of acetate, iso-butyrate, butyrate and valerate (Table 3). Significant decreases of the acetate:propionate ratio was observed at mid and high doses (P < 0·05; Table 3). The lactate concentrations showed no significant difference among treatments (Table 3). However, two animals showed a gradual decline in lactate as they transitioned from control to high BCM (data not shown).
Table 3 SCFA and lactate concentrations (mmol/l) in goats at varying levels of bromochloromethane (BCM)
(Mean values with their standard errors)
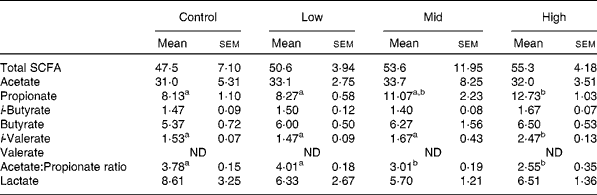
ND, not detected.
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
2H balance calculations
The values of 2H recovered as SCFA are shown in Table 4. Although 28·3–28·7 % of 2HP could be related to SCFA at nil or low BCM dose, more 2HP (32·7–36·0 %) was converted into SCFA at mid or high dose. The 2H accounted for as SCFA relative to the control was increased to 125 % with a high dose of BCM.
Table 4 Metabolic hydrogen produced in rumen fermentation (2HP) incorporated into SCFA
(Mean values with their standard errors, n 3 animals)
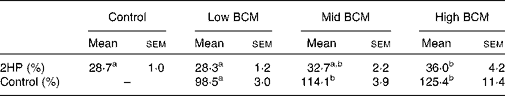
BCM, bromochloromethane.
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
The relationship between methane production (litres/d) and the (C2+C4)/C3 ratio shown in Fig. 2. A highly significant (P < 0·01) linear regression equation was derived as follows
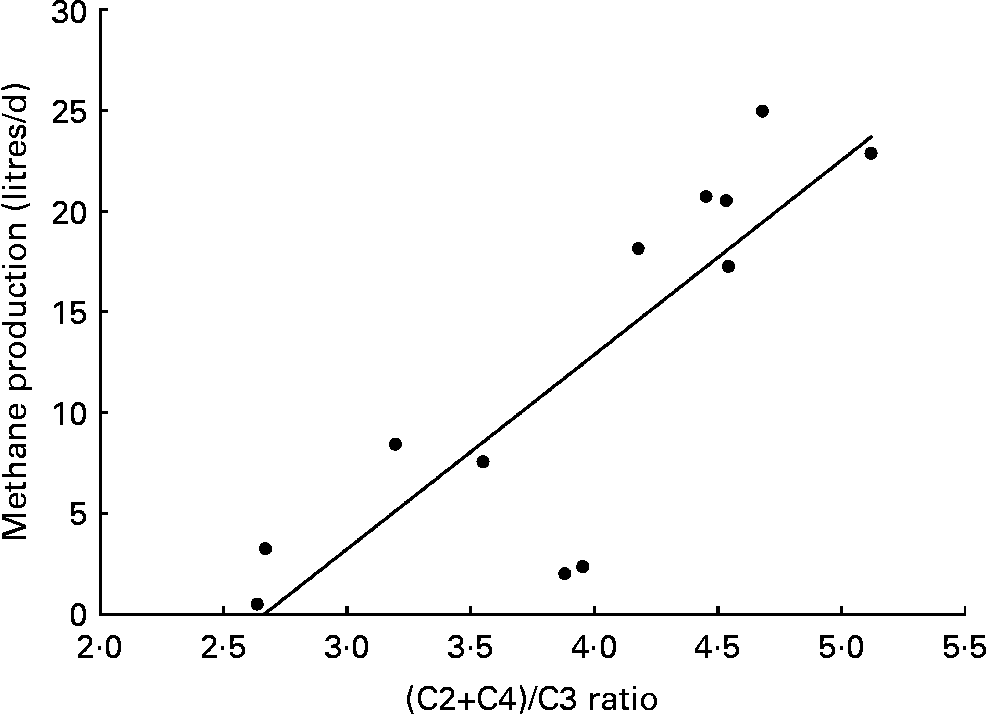
Fig. 2 Relationship between methane production (litres/d) and (C2+C4)/C3 ratio. Methane production was measured using a respiration chamber. (C2+C4)/C3 ratio was determined from concentrations of C2, C3 and C4 in the rumen fluid. y = 9·65x − 25·7, r 2 0·698; P < 0·01.
Simple regression analysis revealed the strong correlation (P < 0·01) between MR level and the percentage of 2HUS, 2HUM or 2HUH/2HU (Fig. 3). It was estimated that the percentage of 2HUH to 2HU increased from 3·1 % at no dose (control) to 55·1 % at high dose.

Fig. 3 Distribution of metabolic hydrogen utilisation (2HU) in SCFA (●), methane (■) or hydrogen (▲) at various levels of bromochloromethane (control, low, mid and high). Observed values of SCFA concentration and methane production were used for calculations of hydrogen production and 2HU.
Monitoring of microbial populations
In the control period, the abundance of methanogens (1·36E+09 ( ± 4·02E+08) copies of 16S rDNA/ml), F. succinogenes (5·98E+08 ( ± 8·22E+07) copies of 16S rDNA/ml), R. albus (1·14E+08 ( ± 1·90E+07) copies of 16S rDNA/ml), R. flavefaciens (8·23E+06 ( ± 2·00E+06) copies of 16S rDNA/ml) and fungal biomass (29·38 ( ± 7·94) μg/ml) was estimated using real-time PCR assays. The methanogen population showed a slight increase in abundance (P < 0·1) for low BCM animals compared with the control and a significant decrease for the mid and high doses (P < 0·5 and P < 0·01, respectively; Fig. 4). At the high BCM dose, the abundance of methanogens decreased by >70-fold.

Fig. 4 Quantitative PCR analysis of methanogens, Fibrobacter succinogenes, Ruminococcus albus, R. flavefaciens and anaerobic fungi population changes in response to doses of bromochloromethane (low, mid and high). a,b Letters denote significant differences from the control period, bars that do not share the same letter for a species are significantly different for each other (P < 0·05). The y-axis denotes fold change from control period.
Populations of fibrolytic bacteria within the rumen were differentially affected by the decline in methanogens and subsequent increase in H2 concentrations. Fibrobacter succinogenes populations increased significantly (P < 0·05) from the control period; however, this may not be biologically significant as it did not equate to at least a 2-fold relative change (Fig. 4). Ruminococcus albus was unaffected by the varying doses of BCM, whereas R. flavefaciens and the anaerobic fungal populations were decreased at all levels of BCM dosage (P < 0·001).
There was no significant change in any of the four protozoal genera identified, because of increases in BCM administration (data not shown). Bacterial community DGGE fingerprints of the 16S rDNA fragments associated with samples collected from goats in each period are shown in Fig. 5. Five distinctive and intense bands, which associated with high BCM, affiliated mainly with Prevotella sp. and a single band with Clostridium aminophilum.

Fig. 5 Denaturing gradient gel electrophoresis (DGGE) patterns of rumen microbial community in three individual goats, A, B and C, to which varying dose of bromochloromethane (BCM; low, mid and high) or no BCM (control) were administrated. 16S rRNA gene fragments were amplified from DNA extracted from rumen samples and loaded onto a DGGE gel. Lane M show markers. The numbered DGGE band indicated by a arrowhead were selected for DNA sequencing.
Prevotella group 1- and 7-specific qPCR primers were designed and validated against full-length 16S rRNA sequences found in the ARB database at the greengenes (http://greengenes.lbl.gov/). Prevotella group 1 comprised sequences from DGGE bands one to three and Prevotella group 7 comprised sequences generated from DGGE bands seven and eight. Prevotella group 1 was most similar (99 %) to the near full-length rumen clone F24-B12 (GenBank accession no. AB185591), whereas Prevotella group 7 primers were most similar (100 %) to a near full-length uncultured rumen bacterium clone L102RC-4-F10 (GenBank accession no. HQ399809). Results from using probe match analysis at Ribosomal database project II and within ARB both showed the primers to be specific for their respective targets. PCR amplification of goat rumen microbial DNA using the specific Prevotella primer sets produced amplicons of expected size. Cloning and sequencing of these amplicons revealed all products examined to align with their respective cluster with a similarity of >98·5 % to each other (data not shown).
For both Prevotella groups, there was a significant increase in numbers as measured by qPCR with respect to BCM dosing, with group 1 increasing by approximately 6-fold at the mid and high dose of BCM and cluster 7 increasing 2·5-fold at the highest BCM dosing compared to the control period (Fig. 6). This was in agreement with the results observed for the increase in intensity for their respect DGGE bands.

Fig. 6 Quantitative PCR analysis of Prevotella groups 1 and 7 population changes in response to doses of bromochloromethane (BCM; low, mid and high). a Letters denote significant differences from the control period, bars that do not share the same letter for a species are significantly different to each other (P < 0·05). The y-axis denotes fold change (log scale) from control period.
Discussion
A dose-dependent inhibitory effect of BCM on the goat rumen was observed for methane production. Doses of net BCM, 0·04, 0·16 and 0·4 g/100 kg LW, reduced methane production by 5, 71 and 91 %, respectively, compared to controls, and there was no effect on maintenance feed intake and neutral-detergent fibre digestibility. This indicates that fibre digestion was not compromised on a highly digestible diet even though H2 concentration increased markedly in the rumen because of inhibition of methanogenesis. Similarly, Goel et al. (Reference Goel, Makkar and Becker12) also showed that BCM did not affect the values for degradability of substrate in an in vitro continuous fermentation study when methanogenesis was inhibited. Moreover, Tomkins et al. (Reference Tomkins, Colegate and Hunter30) reported that DM intake and average daily gain of beef cattle fed a feedlot diet were not influenced by BCM treatment, in which administration of 0·3 g of BCM/100 kg LW reduced almost the same level of methane production, as shown at high dose in the present study.
Methane production in goats was significantly reduced at the mid and high BCM doses similar to other in vivo and in vitro studies. Moreover, the suppression of methane production by BCM led to a large accumulation of H2, which confirms the theoretical predictions by Janssen(Reference Janssen13). H2 accumulation in the rumen has also been reported by Trei et al. (Reference Trei, Parish and Singh31, Reference Trei, Scott and Parish32) in which methane production in the rumen of sheep or lambs was inhibited by administration of 2,2,2-trichloroacetamide or hemiacetal of chloral and starch, respectively. Because the global warming potential of H2 (5·8 over a 100-year time horizon)(Reference Derwent, Simmonds and O'Doherty33) is lower than that of methane (25 over a 100-year time horizon)(Reference Solomon, Qin, Manning, Solomon, Qin, Manning, Chen, Marquis, Averyt, Tignor and Miller34), reducing methane emission is effective for preventing global warming even though H2 may be expired by the animal. It is unlikely that BCM would be used commercially for methane inhibition even though it shows an intensive effect on methane reduction, because this compound is regarded as a greenhouse gas(Reference Solomon, Qin, Manning, Solomon, Qin, Manning, Chen, Marquis, Averyt, Tignor and Miller34). However, it still remains a goal to further enhance the 2HP flows into SCFA in order to reduce production of greenhouse gases and to increase feed efficiency in ruminants.
Shifts in fermentation pathways
Rumen fermentation end products were mostly unchanged with respect to the addition of BCM, but significant increases in propionate and iso-valerate were detected at the mid and high doses. Similar responses have been reported from in vivo and in vitro studies in which BCM was used to inhibit methane formation(Reference Goel, Makkar and Becker12, Reference Denman, Tomkins and McSweeney20). Inhibition of methanogenesis in the rumen is usually associated with an increase in propionate and is believed to be due to the competition for hydrogen(Reference Hungate35). With more hydrogen being available, reductive processes involving propionate production and reductive acetogenesis become thermodynamically favourable(Reference Ungerfeld, Kohn, Sejrsen, Hvelplund and Nielsen2). There was no change in acetate levels, which possibly indicates that reductive acetogenic bacteria have not contributed significantly to the consumption of the accumulated H2. The acetate concentration, however, could reflect a decreased production of acetate by oxidative pathways combined with increased production from reductive acetogenesis. This could be resolved in future studies with the use of labelled substrate required for autotrophic growth of reductive acetogens. The consistent increase in branched chain fatty acids as an adaptive response of the rumen microbiota to BCM may have resulted from proteolytic activity associated with the greater abundance of Prevotella sp.
The balance of reducing equivalents (2H)
It was estimated that about 33–36 % of 2HP in the rumen could be recovered as SCFA at mid and high BCM dose, whereas about 28 % of 2HP was converted into SCFA at the control and low dose. In the in vitro study of Goel et al. (Reference Goel, Makkar and Becker12), the addition of BCM (10 μm) led to a 94 % reduction of methane production and increased the 2H recovered as SCFA from 30·8 % (control) to 36·5 %, which were similar to the recoveries, 28·7 % for control and 36·0 % for high BCM dose, in the present study. Moreover, in their study, as total 2H recovery in SCFA and methane combined for nil BCM (control) and 10 μm BCM treatments were about 93 % and 39 %, respectively, it means 2HP converted into methane changed from 62·2 to 2·5 % by addition of BCM. Similar to Goel et al. (Reference Goel, Makkar and Becker12), methane production was reduced by 91 % and the recovery rate of 2HP into SCFA was significantly increased in the present study, which collectively demonstrates that the rumen microbiota adapted to reduced methanogenesis by redirecting the accumulated 2H into energy-yielding SCFA. Moss et al. (Reference Moss, Jouany and Newbold3) reported that methane production and the ratio (C2+C4)/C3 were highly positively correlated, and presumed that this relationship would be related to the flow rate of hydrogen into SCFA. A significant linear relationship between methane production and the (C2+C4)/C3 ratio was also observed in the present study.
H2 production can be estimated from the ecological stoichiometry of rumen fermentation, with 90 % of 2HP expected to be 2HU (hydrogen recovered as SCFA and methane)(Reference Moss, Jouany and Newbold3, Reference Faichney, Graham and Walker18) under normal conditions. In the present study, 2H recovered in methane and SCFA was calculated to be 40·8 % at high BCM, which was similar to that (38–40 %) of the in vitro study(Reference Goel, Makkar and Becker12). However, even though flow of 2H into SCFA increased by>20 % at the high BCM dose, it was observed and calculated that the majority of 2H available from reduced methane formation flowed into H2 gas instead of SCFA.
Monitoring of microbial populations
The present study has provided the first analysis in vivo of the relationship between total methanogen numbers and level of methane formation from a normal rumen to nearly zero methanogenesis. The methanogen population decreased by 5- and 70-fold at the mid and high BCM doses compared with the controls, which resulted in a 71 and 91 % reduction, respectively, in methane production. This demonstrates that abundance of methanogens is directly related to methane production but not closely correlated, which was observed in a previous related study by Denman et al. (Reference Denman, Tomkins and McSweeney20). Surprisingly, a half-log reduction in the normal methanogen population correlated with>50 % reduction in methane. This probably indicates that the relative methanogenic activity of different archaeal species in the rumen plays a greater role in determining methane output than the absolute number of methanogens.
The population of the highly cellulolytic bacterium F. succinogenes increased slightly in relation to BCM treatment. Unlike the cellulolytic Ruminococci and fungal species in the rumen, F. succinogenes does not produce H2 and is not susceptible to H2 accumulation. Other fibrolytic bacteria and anaerobic rumen fungi rely on interspecies H2 transfer for H2 utilisation and are therefore affected by the loss of the methanogens from this system. Ruminococcus falvefaciens and R. albus are sensitive to partial pressures of hydrogen; increased hydrogen will inhibit NADH oxidation and divert hydrogen to the formation of succinate and ethanol away from acetate(Reference Wolin, Miller, Stewart, Hobson and Stewart36). This is less energetically favourable, and cultures of R. flavefaciens grown without methanogens have a reduced capacity to degrade polysaccharides(Reference Williams, Withers and Joblin37). However, studies of cellulose degradation of R. albus with and without Methanobrevibacter smithii showed a small increase in ATP yield for the co-cultures but no difference in cellulose degradation(Reference Pavlostathis, Miller and Wolin38). These results along with recent fermentation studies of rumen microbial cultures with BCM(Reference Goel, Makkar and Becker12) are in close agreement with the microbial community changes in the present in vivo study, except that a decrease in the fungal population was not observed in the continuous culture system of Goel et al. (Reference Goel, Makkar and Becker12). In addition, anaerobic fungal populations behave in a similar manner to the cellulolytic ruminococci in that, co-culturing with methanogens results in a shift to actetate formation, which is energetically more favourable, and an increase in the rate and extent of cellulose degradation(Reference Bauchop and Mountfort39). The protozoal population did not appear to be affected by BCM even though ecto- and endo-symbiotic methanogens associated with protozoa would have been inhibited. This result is also in agreement with the in vitro fermentation experiments of Goel et al. (Reference Goel, Makkar and Becker12).
On the basis of DGGE analysis, the major shift in bacterial populations in response to BCM was a marked increase in abundance of Prevotella sp. Some Prevotella spp. in the rumen have pathways for propionate production that consume hydrogen via the randomising (succinate) or non-randomising (acrylate) pathways through the fermentation of sugars and lactate, respectively(Reference Bryant, Small and Bouma40–Reference Purushe, Fouts and Morrison42). It is likely that these pathways were the primary routes for consumption of hydrogen, which accumulated as a consequence of reduced methanogenesis. The development of qPCR primers to monitor two of these Prevotella clusters confirmed the observations of the DGGE analysis in that these were dominant bacterial populations that increased in abundance at high BCM dosing levels. However, other bacteria such as Megasphaera elsdenii, Selenomonas ruminantium, Succinimonas amylolytica, Propionibacterium acnes and Veillonella parvula may also be involved, but were not detected by DGGE analysis(Reference Wolin, Miller, Stewart, Hobson and Stewart36, Reference Hungate and Hungate43–Reference Stewart, Flint, Bryant, Hobson and Stewart45). Further studies involving deep DNA-sequencing methodologies combined with metagenomic analysis or tracer experiments with C-labelled sugars and lactate are required to determine the relative contribution of these pathways, as the rumen microbiota adapts to high hydrogen concentration in the rumen.
In conclusion, the present in vivo study in goats showed that inhibition of methanogenesis by>80 % dramatically increased ruminal H2 concentration without affecting DM intake and feed digestibility. A reduction in fibrolytic rumnococci and rumen fungi may have been compensated by a slight increase in Fibrobacter, which prevented an adverse effect on fibre digestion. The methane-inhibited rumen appeared to adapt to the high H2 levels by shifting fermentation to propionate, which was mediated by an increase in the population of hydrogen-consuming Prevotella spp. As the rumen adapted to the high H2 concentration, the flow of metabolic hydrogen into SCFA increased by>20 %, but the majority of 2H (>80 %), which is normally consumed in methane formation, was expelled by the animal. Therefore, consumption of this excess hydrogen into energy-yielding substrates for the animal will require the provision of dietary supplements to drive hydrogen uptake or augmentation of minor hydrogenotrophic pathways such as autotrophic reductive acetogenesis.
Acknowledgements
The present research was funded by a grant from the National Agriculture and Food Research Organisation (NARO), Japan; the Australian Department of Agriculture, Fisheries and Forestry, Meat and Livestock Australia; the Australian Wool Innovation Limited; and Dairy Australia, and also supported by the Japan Society for the Promotion of Science (JSPS) to assemble researchers at the NILGS. The authors gratefully acknowledge the skilled technical assistance of staff of the NILGS Metabolism Section for diligent care of experimental animals and assistance during sample collection. M. M. and C. S. M. designed the research strategy and contributed to the drafting of the manuscript. T. S. and M. M. prepared fistulated goats. A. T. developed the custom-made H2 monitor. O. E., K. H., Y. K., I. N., T. S. and M. M. carried out the respiration chamber analyses. N. A. and S. E. D. designed the qPCR primers and performed the qPCR analyses. T. S. conducted DGGE analyses. M. M., C. S. M., S. E. D. and O. E. performed the data analysis and the statistical analyses. All authors have contributed to the preparation of the manuscript and agree with the submitted manuscript content. The authors declare no conflict of interest.














