Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Pizarro-Sanz, J.L.
Dance, J.M.
Villeneuve, G.
and
Arriortua-Marcaida, M.I.
1994.
The natural and synthetic tavorite minerals: Crystal chemistry and magnetic properties.
Materials Letters,
Vol. 18,
Issue. 5-6,
p.
327.
Rusakov, D. A.
Filaretov, A. A.
Bubentsova, M. N.
Danilov, V. P.
and
Komissarova, L. N.
2006.
Synthesis and crystal structure of the gallium-containing montebrasite analogue LiGa(OH)PO4.
Russian Journal of Inorganic Chemistry,
Vol. 51,
Issue. 6,
p.
852.
Filaretov, A. A.
Zhizhin, M. G.
Khasanov, S. S.
Bobylev, A. P.
Danilov, V. P.
and
Komissarova, L. N.
2006.
Hydrothermal synthesis and crystal structure of a new ammonium gallium hydroxyphosphate (NH4)Ga(OH)PO4.
Russian Journal of Inorganic Chemistry,
Vol. 51,
Issue. 2,
p.
155.
Recham, N.
Chotard, J-N.
Jumas, J-C.
Laffont, L.
Armand, M.
and
Tarascon, J-M.
2010.
Ionothermal Synthesis of Li-Based Fluorophosphates Electrodes.
Chemistry of Materials,
Vol. 22,
Issue. 3,
p.
1142.
Tripathi, Rajesh
Ramesh, T. N.
Ellis, Brian L.
and
Nazar, Linda F.
2010.
Scalable Synthesis of Tavorite LiFeSO4F and NaFeSO4F Cathode Materials.
Angewandte Chemie International Edition,
Vol. 49,
Issue. 46,
p.
8738.
Marx, Nicolas
Croguennec, Laurence
Carlier, Dany
Wattiaux, Alain
Cras, Frédéric Le
Suard, Emmanuelle
and
Delmas, Claude
2010.
The structure of tavorite LiFePO4(OH) from diffraction and GGA + U studies and its preliminary electrochemical characterization.
Dalton Transactions,
Vol. 39,
Issue. 21,
p.
5108.
Song, Hyun‐Kon
Lee, Kyu Tae
Kim, Min Gyu
Nazar, Linda F.
and
Cho, Jaephil
2010.
Recent Progress in Nanostructured Cathode Materials for Lithium Secondary Batteries.
Advanced Functional Materials,
Vol. 20,
Issue. 22,
p.
3818.
Ramesh, T. N.
Lee, Kyu Tae
Ellis, B. L.
and
Nazar, L. F.
2010.
Tavorite Lithium Iron Fluorophosphate Cathode Materials: Phase Transition and Electrochemistry of LiFePO[sub 4]F–Li[sub 2]FePO[sub 4]F.
Electrochemical and Solid-State Letters,
Vol. 13,
Issue. 4,
p.
A43.
Tripathi, Rajesh
Ramesh, T. N.
Ellis, Brian L.
and
Nazar, Linda F.
2010.
Scalable Synthesis of Tavorite LiFeSO4F and NaFeSO4F Cathode Materials.
Angewandte Chemie,
Vol. 122,
Issue. 46,
p.
8920.
Tripathi, Rajesh
Gardiner, Grahame R.
Islam, M. Saiful
and
Nazar, Linda F.
2011.
Alkali-ion Conduction Paths in LiFeSO4F and NaFeSO4F Tavorite-Type Cathode Materials.
Chemistry of Materials,
Vol. 23,
Issue. 8,
p.
2278.
Leblanc, M.
and
Tressaud, A.
2013.
Comprehensive Inorganic Chemistry II.
p.
161.
Julien, Christian M.
Mauger, Alain
and
Groult, Henri
2015.
Advanced Fluoride-Based Materials for Energy Conversion.
p.
77.
2015.
Introduction to Solid State Ionics.
p.
53.
Julien, Christian
Mauger, Alain
Vijh, Ashok
and
Zaghib, Karim
2016.
Lithium Batteries.
p.
269.
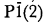 , with refined unit-cell parameters a = 5.340(2), b = 7.283(2), c = 5.110(2)Å, α = 109.29(2)°, β = 97.86(3)°, γ = 106.32(3)°, V = 174.1(3)Å3, a:b:c = 0.7332:1:0.7016, Z = 2, D(m) (suspension in methylene iodide) = 3.32(1) and D(x) = 3.33 g/cm3 (for the theoretical formula). A fully indexed X-ray powder pattern is presented. Semiquantitative electron microprobe and secondary ion mass spectroscopic analyses indicate a formula near end-member LiFe + 3(PO4)(OH). The Tip Top tavorite is biaxial positive, α = 1.795(5), β = 1.81(1), γ = 1.86(1), 2V(meas.) = 50(2)°, 2V(calc.) = 59°, XΛa ≃ 15°, YΛb ≃ 0°, and ZΛc ≃ 38°. There is no evidence for optical absorption, pleochroism or dispersion.
, with refined unit-cell parameters a = 5.340(2), b = 7.283(2), c = 5.110(2)Å, α = 109.29(2)°, β = 97.86(3)°, γ = 106.32(3)°, V = 174.1(3)Å3, a:b:c = 0.7332:1:0.7016, Z = 2, D(m) (suspension in methylene iodide) = 3.32(1) and D(x) = 3.33 g/cm3 (for the theoretical formula). A fully indexed X-ray powder pattern is presented. Semiquantitative electron microprobe and secondary ion mass spectroscopic analyses indicate a formula near end-member LiFe + 3(PO4)(OH). The Tip Top tavorite is biaxial positive, α = 1.795(5), β = 1.81(1), γ = 1.86(1), 2V(meas.) = 50(2)°, 2V(calc.) = 59°, XΛa ≃ 15°, YΛb ≃ 0°, and ZΛc ≃ 38°. There is no evidence for optical absorption, pleochroism or dispersion.



