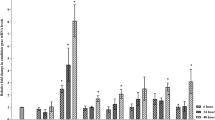
Abstract
Plants have developed mechanisms to successfully co-exist in the presence of pathogenic organisms. Some interactions between plants and pathogens are based on recognition of specific elicitor molecules from avirulent pathogen races (avr gene products), which is described in the gene-for-gene resistance theory. Another type of resistance, multigenic (horizontal) resistance, is a less well-studied phenomenon that depends upon multiple genes in the plant host. All plants possess resistance mechamisms which can be induced upon pre-treatment of plants with a variety of organisms or compounds. This general phenomenon is known as induced systemic resistance (ISR). At least in some plant species, ISR depends on the timely accumulation of multiple gene products, such as hydrolytic enzymes, peroxidases or other gene products related to plant defences. The pre-treatment of plants with an inducing organism or compound appears to incite the plant to mount an effective defense response upon subsequent encounters with pathogens, converting what would have been a compatible interaction to an incompatible one. Our studies in three plant–pathogen systems clearly document that multigenic-resistant plants constitutively express specific isozymes of hydrolytic enzymes that release cell wall elicitors, which in turn may activate other defense mechanisms. ISR induces constitutive accumulation of these and other gene products prior to challenge. ISR is known to function against multiple organisms, and there is no specificity observed in the accumulation patterns of defense-related gene products when ISR is induced. It is therefore hypothesized that the constitutive accumulation of specific isozymes of hydrolytic enzymes, or other defense related gene products, is an integral part of both multigenic resistance and the phenomenon of ISR. Further, plants in which ISR has been activated appear to move from a latent resistance state to one in which a multigenic, non-specific form of resistance is active.
Similar content being viewed by others
References
Abdullah MT (2000) Cloning and characterization of a putative translation initiation factor of Brassica oleracea. PhD dissertation, Auburn University, Auburn, AL, USA
AnfokaGand BuchenauerH(1997) Systemic acquired resistance in tomato against Phytophthora infestans by pre-inoculation with tobacco necrosis virus. Physiol Molec Plant Pathol 1: 351-370
Asiegbu FO, Kacprzak M, Daniel G, Johansson M, Stenlid J a Mañka M (1999) Biochemical interactions of conifer seedling roots with Fusarium spp. Can J Microbiol 45: 923-935
AuhCKand Murphy TM(1995) Plasma membrane redox enzyme is involved in the synthesis of O- 2 and H2O2 by Phytophthora elicitor-stimulated rose cells. Plant Physiol 107: 1241-1247
Bain DC (1952) Reaction of Brassica seedlings to black rot. Phytopathology 42: 497-500
Bain DC (1955) Resistance of cabbage to black rot. Phytopathology 45: 35-37
Barksdale TH and Stoner AK (1973) Segregation for horizontal resistance to tomato early blight. Plant Dis Report 57: 964-965
Benhamou N (1992) Ultrastructural detection of β-1,3-glucans in tobacco root tissues infected by Phytophthora parasitica var. nicotianae using a gold-complexed tobacco β-1,3-glucanase. Physiol Molec Plant Pathol 92: 1108-1120
Benhamou N and Belanger R (1998) Benzothiadiazole-mediated induced resistance to Fusarium oxysporum f. sp. radicislycopersici in tomato. Plant Physiol 118: 1203-1212
Benhamou N, Joosten MHAJ and de Wit PJGM (1990) Subcellular localization of chitinase and of its potential substrate in tomato root tissues infected by Fusarium oxysporum f. sp. radicis-lycopersici. Plant Physiol 92: 1108-1120
Benhamou N, Kloepper JW and Tuzun S (1998) Induction of resistance against Fusarium wilt of tomato by combination of chitosan with an endophytic bacterial strain: ultrastructure and cytochemistry of the host response. Planta 204: 153-168
Benhamou N, Rey P, Chωrif M, Hockenhull J and Tirilly Y (1997) Treatment with the mycoparasite Pythium oligandrum triggers induction of defense-related reactions in tomato roots when challenged with Fusarium oxysporum f. sp. radicis-lycopersici. Phytopathology 87: 108-122
Birecka H, Catalfamo JL and Urban P (1975) Cell wall and protoplast isoperoxidases in tobacco plants in relation to mechanical injury and infection with tobacco mosaic virus. Plant Physiol 55: 611-619
Boller T (1985) Induction of hydrolases as a defense reaction against pathogens. In: Key J and Kosuge T (eds) Cellular and Molecular Biology of Plant Stress (pp 247-262) Alan R. Liss, Inc., New York, NY, USA
Borowicz VA (1997) A fungal root symbiont modifies plant resistance to an insect herbivore. Oecologia 112: 534-542
Brown AEand Davis RD(1992) Chitinase activity in Stylosanthes guianensis systemically protected against Colletotrichum gloeosporoides. J Phytopathol 136: 247-256
Buell CR (1999) Genes involved in plant-pathogen interactions. In: Agrawal AA, Tuzun S, Bent E (eds) Induced Plant Defenses against Pathogens and Herbivores (pp 73-93) APS Press,6St Paul, MN, USA
Busam G, Kassemeyer HH and Matern U (1997) Differential expression of chitinases in Vitis vinifera L. responding to systemic acquired resistance activators or fungal challenge. Plant Physiol 115: 1029-1038
Cohen Y, Niderman T, Mosinger E and Fluhr R (1994) β-aminobutyric acid induces the accumulation of pathogenesis-related proteins in tomato (Lycopersicon esculentum L.) plants and resistance to late blight infection caused by Phytophthora infestans. Plant Physiol 104: 59-66
Cui Y, Bell AA, Joost O and Magill C (2000) Expression of potential defense genes in cotton. Physiol Molec Plant Pathol 56: 25-31
Dann EK, Meuwly P, Mωtraux JP and Deverall BJ (1996) The effect of pathogen inoculation or chemical treatment on activities of chitinase and β-1,3-glucanase and accumulation of salicylic acid in leaves of green bean, Phaseolus vulgaris L. Physiol Molec Plant Pathol 49: 307-319
Dalisay RF and Kuć JA (1995a) Persistence of reduced penetration by Colletotrichum lagenarium into cucumber leaves with induced systemic resistance and its relation to enhanced peroxidase and chitinase activities. Physiol Molec Plant Pathol 47: 329-338
Dalisay RF and Kuć JA (1995b) Persistence of induced resistance and enhanced peroxidase and chitinase activities in cucumber plants. Physiol Molec Plant Pathol 47: 315-327
Dodson KM, Shaw JJ and Tuzun S (1993) Purification of a chitinase/ lysozye isozyme (CHL2) that is constitutively expressed in cabbage varieties resistant to black rot. Abstract A28 Phytopathology 83: 1335
Dubery LA and Slater V (1997) Induced defence responses in cotton leaf disks by elicitors from Verticillium dahliae. Phytochemistry 44: 1429-1434
Elstner EF (1987) Metabolism of active oxygen species. In: Davies DD (ed) The Biochemistry of Plants, Vol 11 (pp 253-315) Academic Press, San Diego, CA, USA
Enkerli J, Gisi U and Mösinger (1993) Systemic acquired resistance to Phythophthora infestans in tomato and the role of pathogenesis-related proteins. Physiol Molec Plant Pathol 43: 161-171
Fought L and Kuć J (1996) Lack of specificity in plant extracts and chemicals as inducers of systemic resistance in cucumber plants to anthracnose. J Phytopathol 144: 1-6
Gardner RG (1988) NC EBR-1 and NC EBR-2 early blight resistant tomato breeding lines. HortScience 23: 779-781
Gay, PA and Tuzun S (2000a) Involvement of a novel peroxidase isozyme and lignification in hydathodes in resistance to black rot disease in cabbage. Can J Bot (in press)
Gay PA and Tuzun S (2000b) Temporal and spatial assessment of defense responses in resistant and susceptible cabbage varieties during infection with Xanthomonas campestris pv. campestris. Physiol Molec Plant Pathol (in press)
Grisebach H (1981) Lignins. In: Conn EE (ed) The Biochemistry of Plants, Vol 7: Secondary Plant Products (pp 457-478) Academic Press, New York, NY, USA
Guzzo SD and Martins EMF (1996) Local and systemic induction of β-1,3-glucanase and chitinase in coffee leaves protected against Hemileia vastatrix by Bacillus thuringiensis hytopathol44: 449-454
Ham KS, Kauffman S, Albetsheim P and Darvill AG (1991) A soybean pathogenesis-related protein with β-1,3-glucanase activity releases phytoalexin elicitor-active heat-stable fragments from fungal walls. Molec Plant Microb Interact 4: 545-552
Hwang SK, Sunwoo JY, Kim YK and Kim BS (1997) Accumulation of β-1,3-glucanase and chitinase isoforms, and salicylic acid in the DL-β-amino-n-butyric acid-induced resistance response of pepper stems to Phytophthora capsici. Physiol Molec Plant Pathol 51: 305-322
Ignatius SMJ, Chopra RK and Muthukrishnan S (1994) Effects of fungal infection and wounding on the expression of chitinases and β-1,3-glucanases in near-isogenic lines of barley. Physiol Plantarum 90: 584-592
Jetiyanon K (1994) Immunization of cabbage for long-term resistance to black rot. MSc Thesis, Auburn University, Auburn, AL, USA
Ju C and Kuć J (1995) Purification and characterization of an acidic β-1,3-glucanase from cucumber and its relationship to systemic disease resistance induced by Colletotrichum lagenarum and tobacco necrosis virus. Molec Plant Microbe Interact 8: 899-905
Karban R and Kuć (1999) Induced resistance against pathogens and herbivores: an overview. In: Agrawal AA, Tuzun S and Bent E (eds) Induced Plant Defenses against Pathogens and Herbivores (pp 1-16) APS Press, St Paul, MN, USA
Keen NT and Yoshikawa M (1983) β-1,3-endoglucanases from soybean release elicito-active carbohydrates from fungus cell walls. Plant Physiol 71: 460-465
Kemp G, Botha AM, Kloppers FJ and Pretorius ZA (1999) Disease development and β-1,3-glucanase expression following leaf rust infection in resistant and susceptible near-isogenic wheat seedlings. Physiol Molec Plant Pathol 55: 45-52
Klement Z (1982) Hypersensitivity. In: Mount MS and Lacy GH (eds) Phytopathogenic Prokaryotes, Vol 2 (pp 149-157) Academic Press, London, UK
Kogel KH, Beckhove U, Dreschers J, Münch S and Rommé Y (1994) Acquired resistance in barley: the resistance mechanism induced by 2,6-dichloroisonicotinic acid is a phenocopy of a genetically based mechanism governing race-specific powdery mildew resistance. Plant Physiol 106: 1269-1277
Krokene P, Christiansen E, Solheim H, Franceschi VR and Berryman AA (1999) Induced resistance to pathogenic fungi in Norway spruce. Plant Physiol 121: 565-569
Lagrimini LM, Gingas V, Finger F, Rothstein S and Liu TFY (1997) Characterization of antisense transformed plants defi-cient in the tobacco anionic peroxidase. Plant Physiol 114: 1187-1196
Lawrence CB, Joosten MHAJ and Tuzun S (1996) Differential induction of pathogenesis-related proteins in tomato by Alternaria solani and the association of a basic chitinase isozyme with resistance. Physiol Molec Plant Pathol 48: 361-377
Lawrence CB, Singh NP, Qiu J, Gardner RG and Tuzun S (2000) Constitutive hydrolytic enzymes are associated with polygenic resistance of tomato to Alternaria solani and may function as an elicitor release mechanism. Physiol Molec Plant Pathol (in press)
Liao YC, Krutzaler F, Fischer R, Reisner JJ and Tizbury R (1994) Characterization of a wheat class Ib chitinase gene differentially induced in isogenic lines by infection with Puccinia graminis. Plant Sci 103: 177-187
Lorito M, Woo SL, Fernandez IG, Colucci G, Harman GE, Pintor-Toro JA, Filippone E, Muccifora S, Lawrence CB, Zonia A, Tuzun S and Scala F (1998) Genes from mycoparasitic fungi as a source for improving plant resistance to fungal pathogens. Proc Natl Acad Sci USA 95: 7860-7865
LussoMandKuć J (1995) Evidence for transcriptional regulation of β-1,3-glucanase as it relates to induced systemic resistance of tobacco to blue mold. Molec Plant Microbe Interact 8: 473-475
Mader Mand Fussi R(1982) Role of peroxidase in lignification of tobacco cells: regulation by phenolic compounds. Plant Physiol 70: 1132-1134.
Mader M, Ungemach J and Schloss P (1980) The role of peroxidase isoenzyme groups of Nicotiana tabacum in hydrogen peroxide formation. Planta 147: 467-470
Maiero M, Ng TJ and Barksdale TH (1990) Inheritance of collar rot resistance in the tomato breeding lines C1943 and NCEBR-2. Phytopathology 80: 1365-1368
Maiero M and Ng TJ (1989) Genetic analyses of early blight resistance in tomatoes. HortScience 24: 221-226.
Manandhar HK, Mathur SB, Smedegaard-Petersen V and Thordal-Christensen H (1999) Accumulation of transcripts for pathogenesis-related proteins and peroxidase in rice plants triggered by Pyricularia oryzae, Bipolaris sorokinana and uv light. Physiol Molec Plant Pathol 55: 289-295
Mauch F, Mauch-Mani B and Boller T (1988) Antifungal hydrolases in pea tissue. II. Inhibition of fungal growth by combinations of chitinases and β-1,3-glucanases. Plant Physiol 88: 936-942
Maurhofer M, Hase C, Meuwly P, Métraux JP and Défago G (1994) Induction of systemic resistance of tobacco to tobacco necrosis virus by the root-colonizing Pseudomonas fluorescens strain CHA0: Influence of the gacA gene and of pyoverdine production. Phytopathology 84: 139-146
McIntyre JL, Dodds JA and Hare JD (1981) Effects of localized infections of Nicotania tabacum by tobacco mosaic virus on systemic resistance against diverse pathogens and an insect. Phytopathology 71: 297-330
Miyazawa J, Kawabata Tand Ogasawara N(1998) Induction of an acidic isozyme of peroxidse and acquired resistance to wilt disease in response to treatment of tomato roots with 2-furoic acid, 4-hydroxybenzoic hydrazide or salicylic hydrazide. Physiol Molec Plant Pathol 52: 115-126
Mosch J and Zeller W (1996) Further studies on plant extracts with a resistance induction effect against Erwinia amylovora. Acta Hort 411: 361-366
Nash AFand Gardner RG(1988)Tomato early blight resistance in a breeding line derived from Lycopersicon hirsutum PI 126445. Plant Dis 72: 206-209
Nelson RR (1978) Genetics of horizontal resistance to plant diseases. Ann Rev Plant Pathol 16: 359-378
Norman C, Vidal S and Palva ET (1999) Oligogalacturonidemediated induction of a gene involved in jasmonic acid synthesis in response to the cell-wall-degrading enzymes of the plant pathogen Erwinia carotovora. Molec Plant Microb Interact 12: 640-644
Pan SQ, Ye XS and Kuć J (1991) Association of β-1,3-glucanase activity and isoform pattern with systemic resistance to blue mould in tobacco induced by stem injection with Peronospora tabacina or leaf inoculation with tobacco mosaic virus. Physiol Molec Plant Pathol 39: 25-39
Pan SQ, Ye XS and Kuć J (1992) Induction of chitinases in tobacco plants systemically protected against blue mold by Peronospora tabacina or tobacco mosaic virus. Phytopathology 82: 119-123
Reimers PJ and Leach JE (1991) Race-specific resistance to Xanthomonas oryzae pv. oryzae conferred by bacterial blight resistance gene xa-10 in rice (Oryza sativa) involves accumulation of a lignin-like substance in host tissues. Physiol Molec Plant Pathol 38: 39-55.
Ride JP (1975) Lignification inwounded wheat leaves in response to fungi and its possible role in resistance. Physiol Plant Pathol 5: 125-134
Robertson TL(1995) Hydrolytic enzyme accumulation in tobacco lines resistant to Peronospora tabacina, the causal agent of blue mold. MSc thesis, Auburn University, Auburn, AL, USA
Rufty RC(1989) Genetics of host resistance to tobacco blue mold. In: McKeen WE (ed) Blue Mold of Tobacco (pp 141-164) APS Press, St Paul, MN, USA
Scandalios JG (1993) Oxygen stress and superoxide dismutases. Plant Physiol 101: 7-12
Schafleitner R and Wilhelm E (1997) Effect of virulent and hypovirulent Cryphonectria parasitica (Murr.) Barr on the intercellular pathogen related proteins and on total protein pattern of chestnut (Castanea stativa Mill.). Physiol Molec Plant Pathol 45: 57-67
Schneider S and Ullrich WR (1994) Differential induction of resistance and enhanced enzyme activities in cucumber and tobacco caused by treatment with various abiotic and biotic inducers. Physiol Molec Plant Pathol 43: 57-67
Sela-Buurlage MB, Ponstein AS, Bres-Vloemans SA, Melchers LS, van der Elzen PMJ and Cornelissen BJC (1993) Only specific tobacco chitinases andβ-1,3-glucanases exhibit antifungal activity. Plant Physiol 101: 857-863
Sharma P, Børja D, Stougaard P and Lönneborg A (1993) PR-proteins accumulating in spruce roots infected with a pathogenic Pythium sp. isolate include chitinases, chitosanases andβ-1,3-glucanases. Physiol Molec Plant Pathol 45: 291-304
Shinshi H, Neuhaus J, Ryals J and Meins F (1990) Structure of a tobacco endochitinase gene: evidence that different chitinase genes can arise by transposition of sequences encoding a cysteine-rich domain. Plant Mol Biol 14: 357-368
Siefert F, Thalmair M, Langbartels C, Sandermann H and Grossman K (1996) Epoxiconazole-induced stimulation of the antifungal hydrolases chitinase and β-1,3-glucanase in wheat. Plant Growth Reg 20: 279-286
Simmonds NW (1991) Genetics of horizontal resistance to diseases of crops. Biol Rev 66: 189-241
Staub T and Williams PH (1972) Factors influencing black rot lesion development in resistant and susceptible cabbage. Phytopathology 62: 722-728
Steijl H, Niemann GJ and Boon JJ (1999) Changes in chemical composition related to fungal infection and induced resistance in carnation and radish investigated by pyrolysis mass spectrometry. Physiol Molec Plant Pathol 55: 297-311
Ton J, Pieterse CJM and van Loon LC (1999) Identification of a locus in Arabidopsis controlling both the expression of a rhizobacteria-mediated induced systemic resistance (ISR) and basal resistance against Pseudomonas syringae pv. tomato. Molec Plant Microb Interact 12: 911-918
Tiburzy R and Reisener HJ (1990) Resistance of wheat to Puccinia graminis f. sp. tritici: association of the hypersensitive reaction with the cellular accumulation of lignin-like material and callose. Physiol Molec Plant Pathol 36: 109-120.
Tuzun S, Gay PA, Lawrence CB, Robertson TB and Sayler RJ (1997) Biotechnological applications of inheritable and inducible resistance to diseases in plants. In: Gresshoff PM(ed) Technology Transfer of Plant Biotechnology (pp 25-40) CRC Press, New York
Tuzun S, Juarez J, Nesmith WC and Kuć J (1992) Induction of systemic resistance in tobacco against metalaxyl-tolerant strains of Peronospora tabacina and the natural occurrence of the phenomenon in Mexico. Phytopathology 82: 425-429
Tuzun S and Kloepper J (1995) Practical application and implementation of induced resistance. In: Hammerschmidt R and Kuć J (eds) Induced Resistance to Disease in Plants: Developments in Plant Pathology. Vol 4 (pp 152-168) Kluwer Academic Publishers, Boston
Tuzun S and Kuć J (1991) Plant immunization: an alternative approach to pesticides for control of plant diseases in the greenhouse and field. In: The Biological Control of Plant Diseases, FFTC Book Series No 42 (pp 30-40) FFTC, Taipei, Taiwan
Tuzun S, Nesmith W, Ferriss RS and Kuć J (1986) Effects of stem injections with Peronospora tabacina on growth of tobacco and protection against blue mold in the field. Phytopathology 76: 938-941
Tuzun S, Rao MN, Vogeli U, Schardl SL and Kuć J (1989) Induced systemic resistance to blue mold: early induction and accumulation ofβ-1,3-glucanases, chitinases and other pathogenesis-related proteins (b-proteins) in immunized tobacco. Phytopathology 79: 979-983
Vad K, Mikkelsen JD and Collinge DB(1991) Induction, purification and characterization of chitinase isolated from pea leaves inoculated with Ascochyta pisi. Planta 184: 24-29
van Loon LC and Callow JA (1983) Transcription and translation in the diseased plant. In: Callow JA (ed) Biochemical Plant Pathology (pp 385-414) John Wiley & Sons, Chichester, UK
van Loon LC and van Strien EA (1999) The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiol Molec Plant Pathol 55: 85-97
Wegener CS, Bartling S, Olsen O, Weber J and von Wertstein D (1996) Pectate lysase in transgenic potatoes confers preactivation of defence against Erwinia carotovora. Physiol Molec Plant Pathol 49: 359-376
Wei ZM and Beer SV (1996) Harpin from Erwinia amylovora induces plant resistance. Acta Hort 411: 223-225
Ye XS, Järlfors, Tuzun S, Pan SQ and Kuć J(1992) Biochemical changes in cell walls and cellular responses of tobacco leaves related to systemic resistance to blue mold (Peronospora tabacina) induced by tobacco mosaic virus. Can J Bot 70: 49-57
Ye XS, Pan SQ and Kuć J (1990) Association of pathogenesisrelated proteins and activities of peroxidase, β-1,3-glucanase and chitinase with systemic induced resistance to blue mould of tobacco but not to systemic tobacco mosaic virus. Physiol Molec Plant Pathol 36: 523-531
Zhang J and Kirkham MB (1994) Drought-stress-induced changes in activities of superoxide dismutase, catalase, and peroxidase in wheat species. Plant Cell Physiol 35: 785-791
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tuzun, S. The Relationship Between Pathogen-induced Systemic Resistance (ISR) and Multigenic (horizontal) Resistance in Plants. European Journal of Plant Pathology 107, 85–93 (2001). https://doi.org/10.1023/A:1008784417222
Issue Date:
DOI: https://doi.org/10.1023/A:1008784417222