Abstract
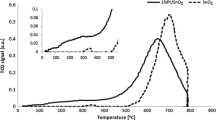
Pure SnO2, sulfated SnO2-SO4 2- and Pd supported on SnO2 and SnO2-SO4 2- were prepared from SnO2 precursor with high surface area, and used for CH4 deep oxidation. The catalysts were characterized by means of N2-BET, XRD, TG-DTA, XPS and TPD. SnO2-SO4 2- shows higher activity than SnO2, due to the presence of more active oxygen species, superacid sites and its higher BET surface area. Pd/SnO2 and Pd/SnO2-SO4 2- display essentially the same activity to each other, while it is much higher than the activity on SnO2 and SnO2-SO4 2-. The main reason is ascribed to the concerted action between Pd and the supports.
Similar content being viewed by others
REFERENCES
Y. Li, J. Armor: Appl. Catal. B, 3, 275 (1994).
Lj. Kundakovic, M. Flytzani-Stephanopoulos: Appl. Catal. A, 183, 35 (1999).
E.S. Rubin, R.N. Cooper, R.A. Frosch, T.H. Lee, G. Marland, A.H. Rosenfield, D.D. Stine: Science, 257, 148 (1992).
M.M. Zwinkel, S.G. Jaras, P.C. Menon: Catal. Rev. Sci. Eng., 35, 319 (1993).
Y. Gao, H. Zhao, B. Zhao: J. Mater. Sci., 35, 917 (2000).
M.J. Fuller, M.E. Warwick: J. Catal., 29, 441 (1973).
G.C. Bond, L.R. Molloy, M.J. Fuller: J. Chem. Soc. Chem. Comm., 796 (1975).
A. Boulahouache, G. Kons, H.G. Lintz, P. Schulz: Appl. Catal. A, 91, 115 (1992).
M.M. Gadgil, R. Sasikala, S.K. Kulshreshtha: J. Mol. Catal., 87, 297 (1994).
K. Sekizawa, H. Widjaja, S. Maeda, Y. Ozawa, K. Eguchi: Appl. Catal. A, 200, 211 (2000).
L. Moens, P. Ruiz, B. Delmon, M. Devillers: Catal. Lett., 46, 93 (1997).
M. C. Kung, P. W. Park, D. W. Kim, H. H. Kung: J. Catal., 181,pp1 (1999).
J. Ma, Y. X. Zhu, J. Y. Wei, X. H. Cai, Y. C. Xie: Stud. Surf. Sci. Catal., 130, pp617 (2000).
X. Song, A. Sayari: Catal. Rev. Sci. Eng., 38, 329 (1996).
F. Lonyi, J. Valyon, J. Engelhardt, F. Mizukami: J. Catal., 160, 279 (1996).
T. M. Jyothi, K. Sreekumar, M. B. Talawar, A. A. Belhekar, B. S. Rao, S. Sugunan: Bull. Chem. Soc. Jpn., 73, 1285 (2000).
W. M. Hua, Z. Gao: Appl. Catal. b, 17, 37 (1998).
X. Wang, Y. C. Xie: React. Kinet. Catal. Lett., 70, 43 (2000).
Y. C. Xie, Y. Q. Tang: Adv. Catal., 37, 1 (1990).
F. Solymosi, J. Raskó, E. Papp, A. Oszko, T. Bánsági: Appl. Catal. A, 131, 55 (1995).
H. Widjaja, K. Sekizawa, K. Eguchi: Bull. Chem. Soc. Jpn., 72, 313 (1999).
M. Machida, K. Eguchi, H. Arai: J. Catal., 103, 385 (1987).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wang, X., Xie, Yc. Low-Temperature CH4 Total Oxidation on Catalysts Based on High Surface Area SnO2. Reaction Kinetics and Catalysis Letters 72, 115–123 (2001). https://doi.org/10.1023/A:1010592800342
Issue Date:
DOI: https://doi.org/10.1023/A:1010592800342