Abstract
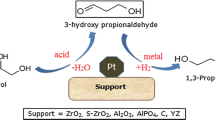
Aqueous-phase reforming of 10 wt% ethylene glycol solutions was studied at temperatures of 483 and 498 K over Pt-black and Pt supported on TiO2, Al2O3, carbon, SiO2, SiO2-Al2O3, ZrO2, CeO2, and ZnO. High activity for the production of H2 by aqueous-phase reforming was observed over Pt-black and over Pt supported on TiO2, carbon, and Al2O3 (i.e., turnover frequencies near 8-15 min-1 at 498 K); moderate catalytic activity for the production of hydrogen is demonstrated by Pt supported on SiO2-Al2O3 and ZrO2 (turnover frequencies near 5 min-1); and lower catalytic activity is exhibited by Pt supported on CeO2, ZnO, and SiO2 (H2 turnover frequencies lower than about 2 min-1). Pt supported on Al2O3, and to a lesser extent ZrO2, exhibits high selectivity for production of H2 and CO2 from aqueous-phase reforming of ethylene glycol. In contrast, Pt supported on carbon, TiO2, SiO2-Al2O3 and Pt-black produce measurable amounts of gaseous alkanes and liquid-phase compounds that would lead to alkanes at higher conversions (e.g., ethanol, acetic acid, acetaldehyde). The total rate of formation of these byproducts is about 1-3 min-1 at 498 K. An important bifunctional route for the formation of liquid-phase alkane-precursor compounds over less selective catalysts involves dehydration reactions on the catalyst support (or in the aqueous reforming solution) followed by hydrogenation reactions on Pt.
Similar content being viewed by others
References
J. Agrell, B. Lindstroem, L. J. Pettersson and S. Jaeras, Catal. Rev. 16 (2002) 67.
R. D. Cortright, R. R. Davda and J. A. Dumesic, Nature (London) 418 (2002) 964.
R. R. Davda, J. W. Shabaker, G. W. Huber, R. D. Cortright and J. A. Dumesic, Appl. Catal. B in press.
J. W. Shabaker, G. W. Huber, R. R. Davda, R. D. Cortright and J. A. Dumesic, J. Catal. in press.
R. R. Davda, R. Alcala, J. W. Shabaker, G. Huber, R. D. Cortright, M. Mavrikakis and J. A. Dumesic, presented at the Fourth Tokyo Conference on Advanced Catalytic Science and Technology, Tokyo (2002).
C. T. Campbell, Chem. Phys. Sol. Surf. 6 (1993) 287.
J. Kaspar, P. Fornasiero and M. Graziani, Catal. Today 50 (1999) 285.
M. Shelef, G. W. Graham and R. W. McCabe, Catal. Sci. Ser. 2 (2002) 343.
N. Takezawa and N. Iwasa, Catal. Today 36 (1997) 45.
G. W. Huber, C. G. Guymon, T. L. Conrad, B. C. Stephenson and C. H. Bartholomew, Stud. Surf. Sci. Catal. 139 (2001) 423.
L. J. Sealock, D. C. Elliott, E. G. Baker and R. S. Butner, Ind. Eng. Chem. Res. 32 (1993) 1535.
K. Ehrhardt, M. Suckow and W. Lutz, Stud. Surf. Sci. Catal. 94 (1995) 179.
C.-H. Chang, R. Gopalan and Y. S. Lin, J. Membr. Sci. 91 (1994) 27.
Y. S. Lin, C.-H. Chang and R. Gopalan, Ind. Eng. Chem. Res. 33 (1994) 860.
M. F. L. Johnson, J. Catal. 123 (1990) 245.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shabaker, J., Huber, G., Davda, R. et al. Aqueous-Phase Reforming of Ethylene Glycol Over Supported Platinum Catalysts. Catalysis Letters 88, 1–8 (2003). https://doi.org/10.1023/A:1023538917186
Issue Date:
DOI: https://doi.org/10.1023/A:1023538917186