Abstract
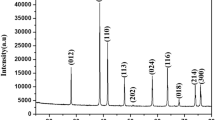
Thermal analysis of some transition metal ferrimaleate precursors, M3[Fe(mal)3]2·xH2O (M=Mn, Co, Ni, Cu) has been studied in static air atmosphere from ambient to 600°C. Various physico-chemical techniques, i.e. TG, DTG, DTA, XRD, IR, Mössbauer spectrometry, have been employed to characterize both the intermediates and final products. After dehydration the anhydrous precursors undergo decomposition to yield an iron(II) intermediate, M[FeII(mal)2] (M=Mn, Co, Ni, Cu) in the temperature range 160-275°C. A subsequent oxidative decomposition of iron(II) species leads to the formation of α-Fe2O3 and MO in the successive stages. Finally a solid-state reaction occurs between the oxides above 400°C resulting in the formation of transition metal ferrites, MFe2O4. The ferrites have been obtained at much lower temperature and in less time than in the conventional ceramic method.
Similar content being viewed by others
References
B. Viswanathan and V. R. K. Murthy, Ferrite Materials, Springer-Verlag, Berlin, 1990.
B. Malecka, E-D. Ciesla, A. Malecki, J. Therm. Anal., 68 (2002) 819.
O. Carp, L. Patron, G. Marinescu, G. Pascu, P. Budrugeac, M. Brezeanu, J. Therm. Anal., 72 (2003) 263.
D. Dollimore and D. L. Griffiths, J. Thermal Anal., 2 (1970) 229.
B. S. Randhawa, J. Mater. Chem., 10 (2000) 2847.
A. I. Vogel, A Text Book of Quantitative Inorganic Analysis including Elementary Instrumental Analysis, Eng. Lang. Book Soc. and Longman, London 1973, p. 786.
K. Nakamoto, Infrared Spectra of Inorganic and Coordination Compounds, John Wiley Intersci., New York 1970.
A. Vertes, L. Korecz and K. Burger, Mössbauer Spectroscopy, Elsevier, New York 1979, p. 47.
A. S. Brar and B. S. Randhawa, J. Phys., 44 (1983) 1385.
M. J. Halsey and A. M. Pritchard, J. Chem. Soc.(A), (1968) 2878.
D. E. Cox, G. Shirane and S. L. Ruby, Phys. Rev., 125 (1962) 1163.
A. H. Morrish, Z. W. Li and X. Z. Zhou, J. Phys. IV, 7 (1997) C1–513.
H. Sato and T. Tominaga, Bull. Chem. Soc. Japan, 55 (1979) 1402.
O. G. Mandada, K. B. Modi, K. M. Jadhav and G. K. Bichile, Indian J. Pure and Appl. Phys., 35 (1997) 554.
B. J. Evans and S. S. Hafner, J. Phys. Chem. Solids, 29 (1968) 1573.
Kirk-Othmer, Encyclopedia of Chemical Technology, 3rd Ed., Vol. 14, Wiley Intersci. Publ., USA, 1981.
B. S. Randhawa and K. J. Sweety, J. Therm. Anal. Cal., 62 (2000) 275.
B. S. Randhawa and K. J. Sweety, J. Radioanal. Nucl. Chem., 247 (2001) 513.
M. J. Ruthner, J. Phys. IV, 7 (1997) C1–53.
G. M. Jeong and S. S. Kim, J. Mag. Soc. Japan, 22 (1998).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Randhawa, B.S., Sweety, K.J., Kaur, M. et al. Synthesis of ferrites: Thermal analysis of some transition metal tris(maleato)ferrates(III). Journal of Thermal Analysis and Calorimetry 75, 101–111 (2004). https://doi.org/10.1023/B:JTAN.0000017333.38139.ff
Issue Date:
DOI: https://doi.org/10.1023/B:JTAN.0000017333.38139.ff