Abstract
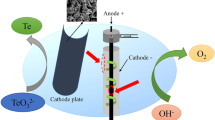
The electrochemical behaviour of tellurium in 2.5 M NaOH solution was studied for the recovery of tellurium from alkaline leach liquor of cemented Te using steady state polarization and cyclic voltammetry. The deposition characteristics and the potential range for a stable deposit of tellurium were also investigated. The morphology of deposited Te in alkaline solution showed a very porous nature and needlelike radial growth. The potential range for stable electrodeposition was between −0.8 V and −0.95 V (vs Hg/HgO electrode), but electrowinning could be carried out at more negative potentials due to the disproportionation reaction of Te2 2−. Laboratory-scale electrowinning experiments were performed under different operating voltages, temperatures and initial Te concentrations. The current efficiency was about 85–90% for 50% recovery and about 50–60% for 90% recovery. The purity of electrodeposited Te was higher than 99.95%.
Similar content being viewed by others
References
E. Hoffmann, JOM 41(7) (1989) 32.
K.-I. Rhee, C.-K. Lee, C.-S. Yoo, T.-H. Kim, H.-S. Kim and H.-J. Sohn, Proceedings of the EPD Congress 1997, TMS, Orlando, FA, 9–13 Feb. (1997), p. 495.
J.J. Lingane and L.W. Niedrach, J. Am. Chem. Soc. 70 (1948) 4115.
J.J. Lingane and L.W. Niedrach, J. Am. Chem. Soc. 71 (1949) 196.
A.J. Panson, J. Phys. Chem. 67 (1963) 2177.
R.A. Jemieson and S.P. Perone, J. Electroanal. Chem. 23 (1969) 411.
K.K. Mishra, D. Ham and K. Rajeshwar, J. Electrochem. Soc. 137 (1990) 3483.
M. Pourbaix, ‘Atlas of Electrochemical Equilibria in Aqueous Solution’, 2nd edn., NACE, Houston TX (1974), p. 560.
G. Trejo, A.F. Gil and I. González, J. Appl. Electrochem. 26 (1996) 1287.
Y. Castrillejo, A.M. Martinez, M. Vega and P.S. Batanero, J. Appl. Electrochem. 26 (1996) 1279.
E. Gómez, J. Ramirez and E. Vallés, J. Appl. Electrochem. 28 (1998) 71.
S.I. Zhdanov, ‘Encyclopedia of Electrochemistry of the Elements’, Vol. 4 (Marcel Dekker, New York, 1974), p. 393.
B. Handle, G. Broderick and P. Paschen, Hydrometallurgy 46 (1997) 105.
J. Jorné and M.C. Lee, J. Electrochem. Soc. 143 (1996) 865.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ha, YC., Sohn, HJ., Jeong, GJ. et al. Electrowinning of tellurium from alkaline leach liquor of cemented Te. Journal of Applied Electrochemistry 30, 315–322 (2000). https://doi.org/10.1023/A:1003867821601
Issue Date:
DOI: https://doi.org/10.1023/A:1003867821601