Abstract
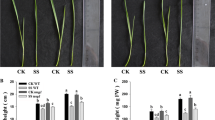
The potential role of photorespiration in the protection against salt stress was examined with transgenic rice plants. Oryza sativa L. cv. Kinuhikari was transformed with a chloroplastic glutamine synthetase (GS2) gene from rice. Each transgenic rice plant line showed a different accumulation level of GS2. A transgenic plant line, G39-2, which accumulated about 1.5-fold more GS2 than the control plant, had an increased photorespiration capacity. In another line, G241-12, GS2 was almost lost and photorespiration activity could not be detected. Fluorescence quenching analysis revealed that photorespiration could prevent the over-reduction of electron transport systems. When exposed to 150 mM NaCl for 2 weeks, the control rice plants completely lost photosystem II activity, but G39-2 plants retained more than 90% activity after the 2-week treatment, whereas G241-12 plants lost these activities within one week. In the presence of isonicotinic acid hydrazide, an inhibitor of photorespiration, G39-2 showed the same salt tolerance as the control plants. The intracellular contents of NH4 + and Na+ in the stressed plants correlated well with the levels of GS2. Thus, the enhancement of photorespiration conferred resistance to salt in rice plants. Preliminary results suggest chilling tolerance in the transformant.
Similar content being viewed by others
References
Asada, K. 1994. Production and action of active oxygen species in photosynthetic tissues. In: C.H. Foyer and P.M. Mullineaux (Eds.), Causes of Photooxidative Stress in Plants and Amelioration of Defense Systems, CRC Press, Boca Raton, FL, pp. 77–104.
Bohnert, H.J., Nelson, D.E. and Jensen, R.G. 1995. Adaptations to environmental stresses. Plant Cell 7: 1099–1111.
Brisson, L.F., Zelitch, I. and Havir, E.A. 1998. Manipulation of catalase levels produces altered photosynthesis in transgenic tobacco plants. Plant Physiol. 116: 259–269.
Burdon, R.H., O'Kane, D., Fadzillah, N., Gill, V., Boyd, P.A. and Finch, R.R. 1996. Oxidative stress and responses in Arabidopsis thaliana and Oryza sativa subjected to chilling and salinity stress. Biochem. Soc. Trans. 24: 468–472.
Fendina, I.S., Tsonev, T.D. and Guleva, E.I. 1994. ABA as a modulator of the response of Pisum sativum to salt stress.J. Plant Physiol. 143: 245–249.
Foyer, C.H., Descourvieres, P. and Kunert, K.J. 1994. Protection against oxygen redicals: an important defence mechanism studied in transgenic plants. Plant Cell Environ. 17: 507–523.
Genty, B., Briantais, J.-M. and Baker, N.R. 1989. The relationship between the quantum yield of photosynthetic transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 990: 87–92.
Gossett, D.R., Millholland, E.P. and Lucas, M.C. 1994. Antioxidant response to salt stress in salt-tolerant and salt-sensitive cultivars of cotton. Crop Sci. 34: 706–714.
Gupta, A.S., Heinen, J.L., Holaday, A.S., Burke, J.J. and Allen, R.D. 1993. Increased resistance to oxidative stress in transgenic plants that overexpress chloroplastic Cu/Zn superoxide dismutase. Proc. Natl. Acad. Sci. USA 90: 1629–1633.
Harris, M.J. and Outlaw, W.H. 1991. Rapid adjustment of guard-cell abscisic acid levels to current leaf-water status. Plant Physiol. 95: 171–173.
Hausler, R.E., Blackwell, R.D., Lea, P.J. and Leegood, R.C. 1994. Control of photosynthesis in barley leaves with reduced activities of glutamine synthetase or glutamate synthase. Planta 194: 406–417.
Hernandez, J.A., Olmos, E., Corpas, F.J., Sevilla, F. and del Rio, L.A. 1995. Salt-induced oxidative stress in chloroplasts of pea plants. Plant Sci. 105: 151–167.
Hibino, T., de Boer, A.D., Weisbeek, P.J. and Takabe, T. 1991. Reconstitution of mature plastocyanin from precursor apoplastocyanin expressed in Escherichia coli. Biochim. Biophys. Acta 1058: 107–112.
Hirel, B. and Gadal, P. 1980. Glutamine synthetase in rice. Plant Physiol. 66: 619–623.
Husic, D.W., Husic, H.D. and Tolbert, N.E. 1987. The oxidative photosynthetic carbon cycle or C2 cycle. CRC Crit. Rev. Plant Sci. 5: 45–100.
Kozaki, A. and Takeba, G. 1996. Photorespiration protects C3 plant from photooxidation. Nature 384: 557–560.
Lee, B.H., Hibino, T., Jo, J., Viale, A.M. and Takabe, T. 1995. Isolation and characterization of dnaK genomic locus in a halotolerant cyanobacterium Aphanothece halophytica. Plant Mol. Biol. 35: 763–775.
Matzke, M.A. and Matzke, J.M. 1995. How and why do plants inactivate homologous (trans) genes. Plant Physiol. 107: 679–685.
McKersie, B.D., Bowley, S.R., Harjanto, E. and Leprince, O. 1996. Water-deficient tolerance and field performance of transgenic alfalfa overexpressing superoxide dismutase. Plant Physiol. 111: 1177–1171.
Murashige, T. and Skoog, F. 1962. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Plant Physiol. 15: 473–497.
Nomura, M., Ishitani, M., Takabe, T., Rai, A.K. and Takabe, T. 1995. Synechococcus sp. PCC7942 transformed with Escherichia coli bet genes produces glycine betaine from choline and acquires resistance to salt stress. Plant Physiol. 107: 703–708.
Ogren, W.L. 1984. Photorespiration: pathways, regulation and modification. Annu. Rev. Plant Physiol. 35: 415–442.
Osmond, C.B. and Grace, S.C. 1995. Perspectives on photoinhibition and photorespiration in the field: quintessential inefficiencies of the light and dark reactions of photosynthesis? J. Exp. Bot. 46: 1351–1362.
Sakamoto, A., Ogawa, M., Masumura, T., Shibata, D., Takeba, G., Tanaka, K. and Fujii, S. 1989. Three cDNA sequences coding for glutamine synthetase polypeptides in Oryza sativa L. Plant Mol. Biol. 13: 611–614.
Schreiber, U., Hormann, H., Neubauer, C. and Klughammer, C. 1995. Assessment of photosystem II photochemical quantum yield by chlorophyll fluorescence quenching analysis. Aust. J. Plant Physiol. 22: 209–220.
Serrano, R. 1996. Salt tolerance in plants and microorganisms: toxicity targets and defense responses. Int. Rev. Cytol. 165: 1–52.
Shimamoto, K., Terada, R., Izawa, T. and Fujimoto, H. 1989. Fertile transgenic rice plants regenerated from transformed protoplasts. Nature 338: 274–276.
Takabe, T., Nakamura, T., Nomura, M., Hayashi, Y., Ishitani, M., Muramoto, Y., Tanaka, A. and Takabe, T. 1998. Glycinebetaine and the genetic engineering of salinity tolerance in plants. In: K. Satoh and N. Murata (Eds.), Stress Responses of Photosynthetic Organisms, Elsevier Science Publishers, Amsterdam, pp.115–131.
Tarczynski, M.C., Jensen, R.G. and Bohnert, H.J. 1993. Stress protection of transgenic tobacco by production of the osmolyte mannitol. Science 259: 508–510.
Volkmar, K.M., Hu, Y. and Steppuhn, H. 1998. Physiological responses of plant to salinity: a review. Can. J. Plant Sci. 78: 19–27.
Wallsgrove, R.M., Turmer, J.C., Hall, N.P., Kendall, A.C. and Bright, S.W. 1987. Barley mutants lacking chloroplast glutamine synthetase. Biochemical and genetic analysis. Plant Physiol. 83: 155–158.
Weatherburn, M.W. 1967. Phenol-hypochlorite reaction for determination of ammonia. Anal. Chem. 39: 971–974.
Willekens, H., Chamnogpol, S., Schraudner, M., Langebartels, C., Van Montagu, M., Inzé, D. and Van Camp, W. 1997. Catalase is a sink for H2O2 and is indispensable for stress defence in C3 plants. EMBO J. 16: 4806–4816.
Wise, R. and Naylor, A.W. 1987. Chilling-enhanced photooxidation. Plant Physiol. 83: 278–282.
Yancey, P.H., Clark, M.E., Hand, S.C., Bowlus, P.D. and Somero, G.N. 1982. Living with water stress: evolution of osmolyte systems. Science 217: 1214–1217.
Zeritch, I. 1980. Measurement of photorespiratory activity and the effect of inhibitors. Meth. Enzymol. 69: 453–464.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hoshida, H., Tanaka, Y., Hibino, T. et al. Enhanced tolerance to salt stress in transgenic rice that overexpresses chloroplast glutamine synthetase. Plant Mol Biol 43, 103–111 (2000). https://doi.org/10.1023/A:1006408712416
Issue Date:
DOI: https://doi.org/10.1023/A:1006408712416