Abstract
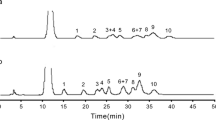
The sugar determination of ulvans, the water-soluble polysaccharides from Ulva sp. and Enteromorpha sp., was optimized by combining partial acid prehydrolysis (2 mol L-1 trifluoroacetic acid, 120°C) with enzymic hydrolysis (with β-D-glucuronidase). The different constitutive sugars (rhamnose, galactose, glucose, xylose, glucuronic acid), released after hydrolysis, were separated by high performance anion-exchange chromatography and determined by pulsed amperometric detection. The ulvanobiouronic acid, β-D-GlcA-(1,4)-L-Rha, which is the main constituent of ulvans was always present after 3 h of trifluoroacetic acid hydrolysis (approx. 2% D.M.) when acid hydrolysis was performed alone but the xylose amount fell to 75% of its maximum value at this time. The optimal duration of 2 mol L−1 trifluoroacetic acid hydrolysis of ulvans (i.e. without any degradation of xylose, rhamnose and glucuronic acid) was 45 min. Additionnal treatment of the partial acid hydrolysate by purified β-D-glucuronidase allowed the hydrolysis of the residual ulvanobiouronic acid in rhamnose and glucuronic acid. High performance anion exchange chromatography coupled to this chemical-enzymic hydrolysis revealed to be a high resolution chromatographic technique for monitoring the hydrolysis of the aldobiouronic acid by β-D-glucuronidase. This method allowed the simultaneous quantitative determination of neutral and acidic sugars and revealed the presence of iduronic acid inulvans.
Similar content being viewed by others
References
Albersheim P, Nevins DJ, English PD, Karr A (1967) A method for the analysis of sugars in plant cell-wall polysaccharides by gas-liquid chromatography. Carbohydr. Res. 5: 340–345.
Arasaki S, Arasaki T (1985) Les Légumes de Mer. Guy Tredaniel, Paris.
BeMiller JN (1967) Acid-catalized hydrolisis of glycosides. Adv. Carbohydr. Chem. 22: 25–108.
Blakeney AB, Harris PJ, Henry RJ, Stone BA (1983) A simple and rapid preparation of alditol acetates for monosaccharide analysis. Carbohydr. Res. 113: 291–299.
Blumenkrantz N, Asboe-Hansen G (1973) New method for the quantitative determination of uronic acids. Analyt. Biochem. 54: 484–489.
Bobin-Dubigeon B, Lahaye M, Barry JL (1997) Human colonic bacterial degradability of dietary fibres from sea-lettuce (Ulva sp). J. Sci. Food Agric. 73: 149–159.
Bradford M (1970) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analyt. Biochem. 72: 248–254.
Chambers RE, Clamp JR (1971) An assessment of methanolysis and other factors used in the analysis of carbohydrate-containing materials. Biochem. J. 125: 1009–1018.
Chaplin MF (1982) A rapid and sensitive method for the analysis of carbohydrate components in glycoproteins using gas-liquid chromatography. Analyt. Biochem. 123: 336–341.
Cheetham NWH, Sirimanne P (1983) Methanolysis studies of carbohydrates, using HPLC. Carbohydr. Res. 112: 1–10.
Fletcher RL (1996) The occurence of ‘green tides'. In Schramm W, Nienhuis PH (eds) Marine Benthic Vegetation — Recent Changes and the Effects of Eutrophication, Springer Verlag, Berlin: 7–43.
Fransson LA (1985) Mammalian glycosaminoglycans. In Aspinall GO (ed.) The Polysaccharides Vol 3, Academic Press, New York: 337–415.
Haug A (1976) The influence of borate and calcium on the gel formation of a sulfated polysaccharide from Ulva lactuca. Acta Chem. Scand. B 30: 562–566.
Hoebler C, Barry JL, David A, Delort-Laval J (1989) Rapid acid hydrolysis of plant cell wall polysaccharides and simplified quantitative determination of their neutral monosaccharides by gas-liquid-chromatography. J. agric. Food Chem. 37: 360–367.
Lahaye M, Axelos MAV (1993) Gelling properties of water-soluble polysaccharides from proliferating marine green seaweeds (Ulva sp). Carbohydr. Polym. 22: 261–265.
Lahaye M, Gomez-Pinchetti JL, Jimenez del Rio M, Garcia-Reina G (1995) Natural decoloration, composition and increase in dietary fibre content of an edible marine algae, Ulva rigida (Chlorophyta). J. Sci. Food Agric. 68: 99–104.
Lahaye M, Jegou D (1993) Chemical and physico-chemical characteristics of dietary fibres from Ulva lactuca (L.) Thuret and Enteromorpha compressa (L.) Grev. J. appl. Phycol. 5: 101–144.
Lahaye M, Ray B (1996) Cell wall frommarine green alga Ulva ‘rigida' (Ulvales, Chlorophyta) — NMR analysis of ulvan oligosaccharides. Carbohydr. Res. 283: 161–173.
Lahaye M, Ray B, Baumberger S, Quemener B, Axelos MAV (1996) Chemical characterisation and gelling properties of cell wall polysaccharides from species of Ulva (Ulvales, Chlorophyta). Hyrobiologia 326/327: 473–480.
Mabeau S, Fleurence J (1993) Seaweed in food products: biochemical and nutritional aspects. Trends Food Sci. Technol. 4: 103–107.
Mulloy B, Forster MJ, Jones C, Drake AF, Johnson EA, Davies DB (1994) The effect of variation of substitution on the solution conforamtion of heparin: a spectroscopic and molecular modelling study. Carbohydr. Res. 255: 1–26.
Percival E, McDowell RH (1967) Chemistry and Enzymology of Marine Algal Polysaccharides. Academic Press, London, UK, 219 pp.
Perlin AS, Casu B, Sanderson GR, Tse J (1972) Methyl α- and β-Didopyranosiduronic acids synthesis and conformational analysis. Carbohydr. Res. 21: 123–132.
Preuss A, Thier HP (1983) Isolierung natürlicher Dickungsmittel aus Lebensmitteln zur cappillar gas ckromatographishen Bestimmung. Z. Lebensm. Unters. Forsch. 176: 5–11.
Quemener B, Lahaye M, Thibault JF (1993) Studies on the simultaneous determination of acidic and neutral sugars of plant cell wall materials by HPLC of their methyl glycosides after combined methanolysis and enzymic prehydrolysis. Carbohydr. Polym. 20: 87–94.
Quemener B, Thibault JF (1990) Assessment of methanolysis for determination of sugars in pectins. Carbohydr. Res. 206: 277–287.
Rao VSR, Balaji PV, Qasba PK (1995) Controversial iduronate ring conformation in dermatan sulphate. Glycobiology 5: 273–279.
Ray B, Lahaye M (1995) Cell wall from marine green alga Ulva ‘rigida' (Ulvales, Chlorophyta) — Chemical structure of ulvan. Carbohydr. Res. 274: 313–318.
Saeman JF, Moore WE, Mitchell RL, Millet MA (1954) Techniques for the determination of pulp constituents by quantitative paper chromatography. Tappi 37: 336–343.
Stevenson TT, Furneaux RH (1991) Chemical methods for the analysis of sulphated galactans from red algae. Carbohydr. Res. 210: 277–298.
Thibault JF (1979) Automatisation du dosage des substances pectiques par la méthode au métohydroxydiphényl. Lebensm.-Wiss. u.-Technol. 12: 247–251.
Venkataraman G, Sasisekharan V, Cooney CL, Langer R, Sasisekharan R (1994) A stereochemical approach to pyranose ring flexibility: its implications for the confirmation of dermatan sulfate. Proc. natl. Acad. Sci. USA 91: 6171–6175.
Whitfield DM, Stojkovski S, Pang H, Baptista J, Sarkar B (1991) Diagnostic methods for the determination of iduronic acid in oligosaccharides. Analyt. Biochem. 194: 259–267.
York WS, Darvill AG, McNeil M, Stevenson TT, Albersheim P (1985) Isolation and characterization of plant cell walls and cell wall components. Methods Enzymol. 118: 3–40.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Quemener, B., Lahaye, M. & Bobin-Dubigeon, C. Sugar determination in ulvans by a chemical-enzymatic method coupled to high performance anion exchange chromatography. Journal of Applied Phycology 9, 179–188 (1997). https://doi.org/10.1023/A:1007971023478
Issue Date:
DOI: https://doi.org/10.1023/A:1007971023478