Abstract
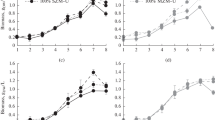
The effects of growth conditions on paramylon (a β-1,3-glucanreserve carbohydrate) content were examined in the photosyntheticwild-type and a spontaneous non-photosynthetic WZSL mutant of theunicellular flagellate Euglena gracilis. This carbohydrate is known toshow interesting applications in human and veterinary medicine, asimmunostimulant and immunopotentiator. For both strains, the synthesisof reserve depends mainly on the growth conditions, i.e. light or dark. Thehighest amount of paramylon is accumulated by the mutant (90% dryweight) under dark conditions and with glucose as the carbon source. Thesefindings are discussed in terms of feasibility of exploitation of both strainsas an alternative source of β-glucan, and of the importance of thechloroplast compartment in the synthesis of this compound.
Similar content being viewed by others
References
Anderson DP, Siwicki AK (1994) Duration of protection against Aeromonas salmonicida in brook trout immunostimulated with glucan or chitosan by injection or immersion. Progres. Fish-Cult. 56: 258-261.
Barras DR, Stone BA (1968) Carbohydrate composition and metabolism in Euglena. In Buetow DE (ed.), The Biology of Euglena, vol. II, Biochemistry. Academic Press, New York, pp. 149-191.
Barsanti L, Bastianini A, Passarelli V, Tredici MR, Gualtieri P (2000) Fatty acid pattern in wild type and WZSL mutant of Euglena gracilis. Effects of carbon sources and growth conditions. J. appl. Phycol. 12.
Bates RC, Hulbert RE (1970) The effect of acetate on Euglena gracilis var. bacillaris as a function of environmental conditions. J. Protozool. 17: 134-138.
Chen D, Ainsworth AJ (1992) Glucan administration potentiates immune defence mechanisms of channel catfish, Ictalurus punctatus Rafinesque. J. Fish Dis. 15: 295-304.
Cramer M, Myers J (1952) Growth and photosynthetic characteristics of Euglena gracilis. Arch. Mikrobiol. 17: 384-402.
Dwyer MR, Smillie RM (1970) A light-induced β-1,3-glucan breakdown associated with the differentiation of chloroplasts in Euglena gracilis. Biochim. Biophys. Acta 216: 392-401.
Efthimiou S (1996) Dietary intake of β-1,3/1,6 glucans in juvenile dentex (Dentex dentex), Sparidae: effects on growth performance, mortalities and non-specific defence mechanisms. J. appl. Ichthyol. 12: 1-7.
Eshleman JN, Danforth WF (1964) Some characteristics of ethanol metabolism in Euglena gracilis var. bacillaris. J. Protozool. 11: 394-399.
Fruehauf JP, Bonnard GD, Herberman RB (1983) The effect of lentinan on production of interleukin-1 by human monocytes. Immunopharmacol. 5: 65-74.
Gottlieb J (1850) Ueber eine neue, mit Starkmehl isomere Substanz. Ann Chem Pharm 75: 51-61.
Gualtieri P, Barsanti L, Passarelli V, Verni F, Rosati G (1990) A look into the reservoir of Euglena gracilis: SEM investigations of the flagellar apparatus. Micr. Microsc. Acta 21: 131-138.
Hayashi M, Toda K, Yoneji T, Sato O, Shozaburo K (1993) Dietary value of rotifers and Artemia enriched with Euglena gracilis for red sea bream. Nippon Suisan Gakkaishi 59: 1051-1058.
Jeney D, Anderson DP (1993) Glucan injection or bath exposure given alone or in combination with a bacterin enhance the non-specific defence mechanism in rainbow trout (Oncorhyncus mykiss). Aquaculture 116: 315-329.
Jorgensen JB, Robertsen B (1995) Yeast β-glucan stimulates respiratory burst activity of Atlantic salmon (Salmo salar L.) macrophages. Develop. Comp. Immunol. 19: 43-57.
Kitaoka S, Hosotani K (1977) Studies on culture conditions for the determination of the nutritive value of Euglena gracilis protein and the general amino acid composition of the cells. Nogei-kagaku 51: 477-482.
Koizumi N, Sakagami H, Utsumi A, Fujinaga S, Takeda M (1993) Anti-HIV (human immunodeficiency virus) activity of sulfated paramylon. Antiv. Res. 21: 1-14.
Kondo Y, Kato A, Hojo H, Nozoe S, Takeuchi M, Ochi K (1992) Cytokine-related immunopotentiating activities of paramylon, a β-1,3-D-glucan from Euglena gracilis. J. Pharmacobio-Dyn. 15: 617-621.
Kusmic C, Barsacchi R, Barsanti L, Gualtieri P, Passarelli V (1999) Euglena gracilis as source of the antioxidant vitamin E. Effects of culture conditions in the wild strain and in the natural mutant WZSL. J appl. Phycol. 10: 555-559.
Murakami T, Ogawa H, Hayashi M, Yoshizumi H (1988) Effect of Euglena cells on blood pressure, cerebral and peripheral vascular changes and life-span in stroke-prone spontaneously hypertensive rats. J. Jpn. Soc. Nutr. Food Sci. 41: 115-125.
Osumi M (1998) The ultrastructure of yeast: cell wall structure and formation. Micron 29: 207-233.
Quesada LA, De Lustig ES, Marechal LR, Belocopitow E (1976) Antitumor activity of paramylon on sarcoma-180 in mice. Gann 67: 455-459.
Rosati G, Barsanti L, Passareli V, Giambelluca A, Gualtieri P (1996) Ultrastructure of a novel non-photosynthetic Euglena mutant. Micron 27: 367-373.
Schwartzbach SD, Schiff JA, Neil HD (1975) Events surroundings the early development of Euglena chloroplasts. V. Control of paramylon degradation. Plant Physiol. 56: 313-317.
Sugawara I, Lee KC, Wong M (1984) Shizophyllan (SPG)-treated macrophages and anti-tumour activities against syngeneic and allogeneic tumour cells. I. Characteristics of SPG-treated macrophages. Cancer Immunol. Immunother. 16: 137-144.
Sung HH, Kou GH, Song YL (1994) Vibriosis resistance induced by glucan treatment in tiger shrimp (Penaeus monodon) Fish Pathology 29: 11-17.
Takeyama H, Kanamaru A, Yoshino Y, Kakuta H, Kawamura Y, Matsunaga T (1997) Production of antioxidant vitamins, β-carotene, vitamin C, vitamin E, by two-step culture of Euglena gracilis Z. Biotech. Bioengng 53: 185-190.
Wang L, Behr SR, Newman RK, Newman CW (1997) Comparative cholesterol-lowering effects of barley β-glucan and barley oil in golden syrian hamsters. Nutrition Res. 17: 77-88.
Wood PJ (1994) Evaluation of oat bran as a soluble fibre source. Characterization of oat β-glucan and its effects on glycaemic response. Carb. Pol. 25: 331-336.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Barsanti, L., Vismara, R., Passarelli, V. et al. Paramylon (β-1,3-glucan) content in wild type and WZSL mutant of Euglena gracilis. Effects of growth conditions. Journal of Applied Phycology 13, 59–65 (2001). https://doi.org/10.1023/A:1008105416065
Issue Date:
DOI: https://doi.org/10.1023/A:1008105416065