Abstract
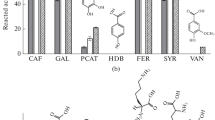
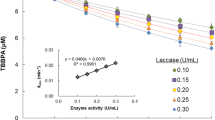
The herbicide bentazon (3-isopropyl-1H-2,1,3-benzothiadiazine-4(3 H)-one-2,2-dioxide), a relatively inert chemical, and some of its metabolites were incubated with a laccase or a peroxidase in the presence or absence of humic monomers to evaluate the incorporation of the herbicide and its metabolites into humic material by oxidative enzymes. Guaiacol and ferulic acid were used as representative electron donor co-substrates in most of the oxidative coupling reactions. Bentazon and its metabolites, with the exception of hydroxy metabolites, underwent little or no transformation by the two enzymes in the absence of guaiacol and ferulic acid,but in the presence of these co-substrates transformation occurred. The reaction of bentazon with guaiacol in the presence of the laccase or a peroxidase was almost complete in30 min. 6-Hydroxy- and 8-hydroxy-bentazon were completely transformed by each enzyme both with and with out co-substrates. At pH 3.0 and in the presence of laccase and guaiacol, the concentrations of bentazon and its metabolites2-amino-N-isopropyl-benzamide (AIBA), des-isopropyl-bentazon and 8-chloro-bentazon decreased by 27, 57, 20 and 4%,respectively. The corresponding levels of transformation with peroxidase at pH 3.0 were 9, 70, 30 and 5%, respectively. The extent of transformation decreased with increasing pH. At low pH, the hydroxy-bentazons were completely transformed,followed by (in order of percentage transformation) AIBA,des-isopropyl-bentazon, bentazon and 8-chloro-bentazon. Transformation of bentazon by the laccase increased with increasing guaiacol concentration. In the presence of the peroxidase, the most effective co-substrates for transformation of bentazon were (in decreasing order) catechol, vanillicacid, protocatechuic acid, syring aldehyde and caffeic acid,while in the presence of the laccase, catechol was most effective, followed by caffeic acid, protocatechuic acid and syringaldehyde.
Similar content being viewed by others
References
Bollag JM & Myers C (1992) Detoxification of aquatic and terrestrial sites through binding of pollutants to humic substances. Sci. Total Environ. 117/118: 357–366
Bollag JM, Myers CJ, Pal S & Huang PM(1995) The role of abiotic and biotic catalysts in the transformation of phenolic compounds. In: Huang PM, Berthelin J, Bollag JM, McGill WB & Page AL (Eds) Environmental Impact of Soil Component Interactions (pp 299–309). CRC Press, Inc., Boca Raton, Florida
Bollag JM, Myers CJ & Minard RD (1992) Biological and chemical interactions of pesticides with soil organic matter. Sci. Total Environ. 123/124: 205–217
Dec J & Bollag JM (1995) Application of plant materials for the cleanup of wastewater. In: Hinchee RE, Leeson A & Semprini L (Eds) Bioremediation of Chlorinated Solvents (pp 307–312). Battelle Press, Columbus, Ohio
Dec J & Bollag JM (1990) Detoxification of substituted phenols by oxidoreductive enzymes through polymerization reactions. Arch. Environ. Contam. Toxicol. 19: 543–550
EbertD(1992) Einfluss von Regenw¨urmern auf die Bioverf¨ugbarkeit vonBentazon und seiner Abbauprodukte imBoden. Ph.D. Thesis, Natural Science Department, RuprechtKarlsUniversity, Heidelberg
Huber R & Otto S (1994) Environmental behavior of bentazon herbicide. Rev. Environ. Contam. Toxicol. 137: 111–134
Kim JE, Park JW, Ebert D & Bollag JM (1998) Distribution of bound 14Cbentazon in soil under laboratory conditions (in preparation)
Klibanov AM, Tu TM & Scott KP (1983) Peroxidasecatalyzed removal of phenols from coalconversion waste waters. Science 221: 259–261
Lee JK, F¨uhr F & Mittelstaedt W (1988) Formation and bioavailability of bentazon residues in German and Korean agricultural soil. Chemosphere 17: 441–450
Otto S, Beutel P, Drescher N & HuberR(1978) Investigations into the degradation of bentazon in plants and soil. IUPAC, Advances in Pesticide Science (Zurich 1978) (pp 551–556). Pergamon Press, Oxford
Roper JC, Sarkar JM, Dec J & Bollag JM (1995) Enhanced enzymatic removal of chlorophenols in the presence of cosubstrates. Wat. Res. 29: 2720–2724
Ruggiero P, Dec J & Bollag JM (1996) Soil as a catalytic system. In: Stotzky G & Bollag JM (Eds) Soil Biochemistry, Vol. 9 (pp 79–122). Marcel Dekker, Inc., New York
Shindo H & Huang PM (1982) Role of Mn (IV) oxide in abiotic formation of humic substances in the environment. Nature (London) 298: 363–365
Simmons KE, Minard RD & Bollag JM (1988) Oxidative coupling and polymerization of guaiacol, a lignin derivative. Soil Sci. Soc. Am. J. 52: 1356–1360
Sparks DL (1995) Environmental Soil Chemistry, Academic Press, New York pp 53–79
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kim, JE., Wang, Cj.J. & Bollag, JM. Interaction of reactive and inert chemicals in the presence of oxidoreductases: Reaction of the herbicide bentazon and its metabolites with humic monomers. Biodegradation 8, 387–392 (1997). https://doi.org/10.1023/A:1008206210134
Issue Date:
DOI: https://doi.org/10.1023/A:1008206210134