Abstract
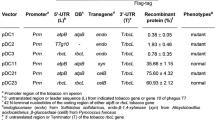
The economical bioconversion of lignocellulosic biomass to ethanol is dependent on the availability of large quantities of inexpensive cellulase enzymes. One way to reduce the cost of such enzymes is to produce them in crop plants at high levels. In order to assess factors that limit recombinant cellulase expression in plants, we have introduced the gene encoding E1 endo-1,4-β-glucanase (cellulase) of Acidothermus cellulolyticus into tobacco (Nicotiana tabacum) plants. Both the holoenzyme (E1) and catalytic domain (E1cd) were targeted to three sub-cellular compartments; the cytosol, chloroplast and apoplast. Accumulation of both E1 and E1cd was greatest in the apoplast, with levels more than 100-fold higher than observed for cytosolic accumulation. In all three compartments, E1cd accumulated to higher levels than the full-length enzyme. By combining truncation and apoplastic localization, an increase in expression of more than 500-fold was achieved, compared to cytosolic full-length E1. This effect is primarily post-transcriptional, since E1cd mRNA levels are very similar despite the range of E1cd accumulation observed. Recombinant E1cd, expressed at up to 1.6% total soluble protein, is extremely stable in both crude leaf extracts and dried leaf material.
Similar content being viewed by others
References
Austin S., Bingham E.T., Mathews D.E., Shahan M.N., Will J. and Burgess R.R. 1995. Production and field performance of transgenic alfalfa (Medicago sativa L.) expressing α-amylase andmanganese-dependent lignin peroxidase. Euphytica 85: 381–393.
Austin-Phillips S., Koegel R., Straub R. and Cook M. 1998. Animal feed compositions containing phytase derived from transgenic alfalfa and methods of use thereof. US patent 5,900,525.
Austin-Phillips S., Burgess R., Ziegelhoffer T. and German T.L. 1999. Transgenic plants as an alternative source of lignocellulosic degrading enzymes. US patent 5,981,835.
Ausubel F.M., Brent R., Kingston R.E., Moore D.D., Seidman J.G., Smith J.A. and Struhl K. 1989. Short Protocols in Molecular Biology. John Wiley, New York.
Baker J.O., Adney W.S., Nieves R.A., Thomas S.R., Wilson D.B. and Himmel M.E. 1994. A new thermostable endoglucanase, Acidothermus cellulolyticus E1. Synergism with Trichoderma reesei CBH I and comparison to Thermomonospora fusca E5. Appl Biochem. Biotechnol. 45/46: 245–256.
Berry-Lowe S.L., McKnight T.D., Shah D.M. and Meagher R.B. 1982. The nucleotide sequence, expression, and evolution of one member of a multigene family encoding the small subunit of rubulose-1,5-bisphophate carboxylase in soybean. J. Mol. Appl. Genet. 1: 483–498.
Boston R.S., Viitanen P.V. and Vierling E. 1996. Molecular chaperones and protein folding in plants. Plant Mol. Biol. 32: 191–222.
Cheung W.Y., Nathalie H. and Landry B.S. 1993. A simple and rapid DNA microextraction method for plant, animal and insect suitable for RAPD and other PCR analyses. PCR Meth. Appl. 3: 69–70.
Conrad U. and Fiedler U. 1998. Compartment-specific accumulation of recombinant immuno-globulins in plant cells: an essential tool for antibody production and immunomodulation of physiological functions and pathogen activity. Plant Mol. Biol. 38: 101–109.
Creissen G., Reynolds H., Xue Y. and Mullineaux P. 1995. Simultaneous targeting of pea glutathione reductase and of a bacterial fusion protein to chloroplasts and mitochondria in transgenic tobacco. Plant J. 8: 167–175.
Dai Z., Hooker B.S., Quesenberry R.D. and Gao J. 1999. Expression of Trichoderma reesei exo-cellobiohydrolase I in transgenic tobacco leaves and calli. Appl. Biochem. Biotechnol. 77-79: 689–699.
Dai Z., Hooker B.S., Anderson D.B. and Thomas S.R. 2000a. Expression of Acidothermus cellulolyticus endoglucanase E1 in transgenic tobcco: biochemical characteristics and physiological effects. Transgenic Res. 9: 43–54.
Dai Z., Hooker B.S., Anderson D.B. and Thomas S.R. 2000b. Improved plant-based production of E1 endoglucanase using potato: expression optimization and tissue targeting. Mol. Breed. 6: 277–285.
Deragon J.M. and Landry B.S. 1992. RAPD and other PCR-based analyses of plant genomes. PCR Meth. Appl. 1: 175–180.
Halliwell N. and Halliwell G. 1995. Biotechnological aspects of lignocellulose and biomass degradation. Outlook Agric. 24: 219–225.
Haseloff J., Siemering K.R., Prasher D.C. and Hodge S. 1997. Removal of a cryptic intron and sub-cellular localization of green fluorescent protein are required to mark transgenic Arabidopsis plants brightly. Proc. Natl. Acad. Sci. USA 94: 2122–2127.
Heber U., Pon N.G. and Heber M. 1963. Localization of carboxydismutase & triosephosphate dehydrogenases in chloroplasts. Plant Physiol. 38: 355–360.
Hendriks T., van den Berg B.M. and Schram A.W. 1985. Cellular location of peroxidase isoenzymes in leaf tissue of Petunia and their affinity for concanavalin A-Sepharose. Planta 164: 89–95.
Jefferson R.A., Kavanagh T.A. and Bevan M.W. 1987. GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 6: 3901–3907.
Koegel R., Straub R. and Austin-Phillips S. 1998. Phytase-proteinpigmenting concentrate derived from green plant juice. [Author: please mention source].
Koegel R.G., Straub R.J., Austin-Phillips S., Cook M.E. and Crenshaw T.D. 1999. Alfalfa-produced phytase for supplementation of poultry and swine rations. In: International Summer Meeting of the American Society of Agricultural Engineers.
Koziel M.G., Carozzi N.B. and Desai N. 1996. Optimizing expression of transgenes with an emphasis on post-transcriptional events. Plant Mol. Biol. 32: 393–405.
Levy M. and Adam Z. 1995. Mutations in the processing site of the precursor of rubulose-1,5-carboxylase/oxygenase small subunit: effects on import, processing, assembly and stability. Plant Mol. Biol. 29: 53–61.
Lynd L.R., Cushman J.H., Nichols R.J. and Wyman C.E. 1991. Fuel ethanol from cellulosic biomass. Science 251: 1318–1323.
Nishimura M., Graham D. and Akazawa T. 1976. Isolation of intact chloroplasts and other cell organelles from spinach leaf protoplasts. Plant Physiol. 58: 309–314.
Parvathi K., Bhagwat A.S. and Raghavendra A.S. 1998. Modulation by bicarbonate of catalytic and regulatory properties of C4 phosphoenolphyruvate carboxylase from Amaranthus hypochondriacus: desensitization to malate and glucose 6-phosphate and sensitization of Mg2+. Plant Cell Physiol. 39: 1294–1298.
Rathmell W.G. and Sequeira L. 1974. Soluble peroxidases in fluid from intercellular spaces in tobacco leaves. Plant Physiol. 53: 317–318.
Sakon J., Adney W.S., Himmel M.E., Thomas S.R. and Karplus P.A. 1996. Crystal structure of thermostable family 5 endocellulase E1 from Acidothermus cellulolyticus in complex with cellotetraose. Biochemistry 35: 10648–10660.
Sheehan J. and Himmel M. 1999. Enzymes, energy, and the environment: a strategic perspective on the U.S. Department of Energy's research and development activities for bioethanol. Biotech. Prog. 15: 817–827.
Shepard J.F. and Totten R.E. 1975. Isolation and regeneration of tobacco mesophyll cell protoplasts under low osmotic conditions. Plant Physiol. 55: 689–694.
Sullivan M.L. and Green P.J. 1993. Post-transcriptional regulation of nuclear-encoded genes in higher plants: the roles of mRNA stability and translation. Plant Mol. Biol. 23: 1091–1104.
Teeri T.T. 1997. Crystalline cellulose degradation: new insight into the function of cellobiohydrolases. Trends Biotechnol. 15: 160–167.
Tucker M.P., Mohegheghi A., Grohmann K. and Himmel M.E. 1989. Ultra-thermostable cellulases from Acidothermus cellulolyticus: comparison of temperature optima with previously reported cellulases. Bio/technology 7: 817–820.
Wong E.Y., Hironaka C.M. and Fischhoff D.A. 1992. Arabidopsis thaliana small subunit leader and transit peptide enhance the expression of Bacillus thuringiensis proteins in transgenic plants. Plant Mol. Biol. 20: 81–93.
Ziegelhoffer T., Will J. and Austin-Phillips S. 1999. Expression of bacterial cellulase genes in transgenic alfalfa (Medicago sativa L.), potato (Solanum tuberosum L.) and tobacco (Nicotiana tabacum L.). Mol. Breed. 5: 309–318.
Ziegler M.T., Thomas S.R. and Danna K.J. 2000. Accumulation of a thermostable endo-1,4-β-D-glucanase in the apoplast of Arabidopsis thaliana leaves. Mol. Breed. 6: 37–46.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ziegelhoffer, T., Raasch, J.A. & Austin-Phillips, S. Dramatic effects of truncation and sub-cellular targeting on the accumulation of recombinant microbial cellulase in tobacco. Molecular Breeding 8, 147–158 (2001). https://doi.org/10.1023/A:1013338312948
Issue Date:
DOI: https://doi.org/10.1023/A:1013338312948