Abstract
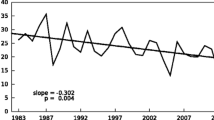
We have observed acid fog in Mt. Oyama since 1988. Fog events occur frequently in Mt. Oyama; 47% of the time the mountain top is covered with fog. The pH of fog is lower than that of rain collected at the fog sampling station by about 1 unit and the lowest pH was 1.95. We have also collected gas and aerosol components at the station and observed fog events by a video camera from the mountain base, where there is an air pollution monitoring station. The air quality at the fog sampling station was affected by not only the air quality at the base but also the wind direction, valley or mountain wind. It is ascertained by using back trajectory analyses that polluted air masses are transported to the base from the Kanto plains or other big urban areas, Osaka and Nagoya. When the base is polluted, the relative humidity increases, and the valley wind blows, acid fog is formed at the mountainside. In the acid fog, nitrate ion is the most abundant anion. Nitrate ion concentration correlates well with hydrogen ion concentration. The annual mean total ion concentration has not changed much since 1988, but the concentration ratio of nitrate ion to sulfate ion has increased in recent years. The nitric acid gas concentration in the station is comparable to that in urban areas, while other gas concentrations are much lower.
Similar content being viewed by others
References
Facchini, M. C., Fuzzi, S., and Orsi, G.: 1998, The NEVALPA Project: Fog Chemical Climatology over the Po Valley Basin. Proceedings of International Conference on Fog and Fog Collection, Vancouver, Canada.
Hosono, T., Okochi, H., and Igawa, M.: 1994, Bull. Chem. Soc. Jpn., 67, 368.
Igawa, M., Hoka, E., Hosono, T., Iwase, K., and Nagashima, T.: 1991, Nippon Kagaku Kaishi (J. Chem. Soc. Jpn.), 698.
Igawa, M., Kameda, H., Maruyama, F., Okochi, H., and Otsuka, I.: 1997, Environ. Exp. Bot., 38, 155.
Igawa, M., Tsutsumi, Y., Mori, T., and Okochi, H.: 1998, Environ.Sci.Technol., 32, 1566.
Jagels, R., Cunningham, R., Carlisle, J., and Day, M., 1998, Documented 50–year Change In Acidity and Chemistry of New England Fog. Proceedings of International Conference on Fog and Fog Collection, Vancouver, Canada.
Mori, K., Okochi, H., and Igawa, M.: 1997, J. Jpn. Soc. Air Pollut., 32, 157.
Okanoue, M. and Ohtani, Y.: 1981, Shinrin Ricchi, 23, 31.
Saxena, V. K. and Lin, N.-H.: 1990, Atmos. Environ., 24A, 329.
Suzuki, K.: 1992, Bull. Kanagawa Prefecture Forest Exp. Sta., 19, 23.
Vong, R. J., Sigmon, J. T., and Mueller, S. F.: 1991, Environ. Sci. Technol., 25, 1014.
Waldman, J. M., Munger, J. W., Jacob, D. J., Flagan, R. C., Morgan, J. J., Hoffman, M. R.: 1982, Science, 218, 677.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Igawa, M., Matsumura, K. & Okochi, H. Fog Water Chemistry at Mt. Oyama and its Dominant Factors. Water, Air, & Soil Pollution 130, 607–612 (2001). https://doi.org/10.1023/A:1013867620159
Issue Date:
DOI: https://doi.org/10.1023/A:1013867620159