Abstract
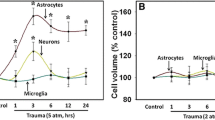
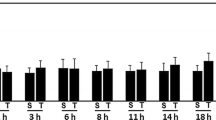
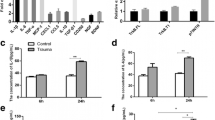
CNS trauma has been associated with an increase in free radical production, but the cellular sources of this increase or the mechanism involved in the production of free radicals are not known. We, therefore, investigated the effects of trauma on free radical production in cultured neurons, astrocytes and BV-2 microglial cells. Free radicals were measured with the fluorescent dye DCFDA following in vitro trauma. At 30 and 60 min following trauma, there was a 132% and 64% increase, respectively, in free radical production in neurons when compared to controls. In astrocytes, there was a 94% and 133% increase at 30 and 60 min, respectively. Microglial cells, however, displayed no significant increase in free radicals at 30, 60 or 120 min following trauma. Since trauma can induce the mitochondrial permeability transition (MPT), a process associated with mitochondrial dysfunction, we further investigated whether cyclosporin A (CsA), an agent known to block the MPT, could prevent free radical formation following trauma. In neurons CsA did not block free radical production at 30 min but blocked it by 90% at 60 min. In contrast, in astrocytes CsA completely blocked free radical production at 30 min but did not block it at 60 min. Our results indicate that a differential sensitivity to trauma-induced free radical production exists in neural cells; that the MPT may be involved in the production of free radical post-trauma; and that the CsA-sensitive phase of free radical production is different in neurons and astrocytes.
Similar content being viewed by others
REFERENCES
Kontos, H. A. and Povlishok, J. T. 1986. Oxygen radicals in brain injury. Central Nervous System Trauma 3:257–263.
Braughler, J. M. and Hall, E. D. 1989. Central nervous system trauma and stroke. I. Biochemical consideration for oxygen radical formation and lipid peroxidation. Free Rad. Biol. Med. 6:289–301.
Hall, E. D. 1993. The role of oxygen radicals in traumatic injury: Clinical implications. J. Emergency Med. 11:31–36.
Hall, E. D., Andrus, P. K., and Yonkers, P. A. 1993. Brain hydroxyl radical generation in acute experimental head injury. J. Neurochem. 60:588–594.
Lewen, A., Matz, P., and Chan, P. K. 2000. Free radical pathways in CNS injury. J. Neurotrauma 17:871–890.
Sen, S., Goldman, H., Morehead, M., Murphy, S., and Phillis, J. W. 1993. Oxypurinol inhibits free radical release from the cerebral cortex of closed head injured rats. Neurosci. Lett. 162:117–120.
Sen, S., Goldman, H., Morehead, M., Murphy, S., and Phillis, J. W. 1993. alpha-Phenyl-tert-butyl-nitrone inhibits free radical release in brain concussion. Free Radic. Biol. Med. 16:685–691.
Sullivan, P. G., Thompson, M. B., and Scheff, S. W. 1999. Cyclosporin A attenuates acute mitochondrial dysfunction following traumatic brain injury. Exp. Neurol. 160:226–234.
Shepard, S. R., Ghajar, J. B. G., Gianuzzi, R., Kupferman, S., and Hariri, R. J. 1991. Fluid percussion barotrauma chamber: a new in vitro model for traumatic brain injury. J. Surg. Res. 51:417–424.
Fiskum, G. 2000. Mitochondrial participation in ischemic and traumatic neural cell death. J. Neurotrauma 17:843–855.
Nilsson, P., Hillered, L., Ponten, U., and Ungerstedt, U. 1990. Changes in extracellular levels of energy-related metabolites and amino acids following concussive brain injury to rats. J. Cereb. Blood Flow Metab. 10:631–637.
Vink, R., Head, V. A., Rodgers, P. J., McIntosh, T. K., and Faden, A. I. 1990. Mitochondrial metabolism following traumatic brain injury in rats. J. Neurotrauma 7:21–27.
Bernardi, P., Vassanelli, S., Veronese, P., Colonna, R., Szabo, I., and Zoratti, M. 1992. Modulation of the mitochondrial permeability transition pore. Effect of protons and divalent cations. J. Biol. Chem. 267:2934–2939.
Bernardi, P., Colonna, R., Costantini, P., Eriksson, O., Fontaine, E., Ichas, F., Massari, S., Nicolli, A., Petronilli, V., and Scorrano, L. 1998. The mitochondrial permeability transition. Bio-Factors. 8:273–281.
Kroemer, G. and Reed, J. C. 2000. Mitochondrial control of cell death. Nature Medicine. 6:513–519.
Lemasters, J. J., Nieminen, A. L., Qian, T., Trost, L. C., Elmore, S. P., Nishimura, Y., Crowe, R. A., Cascio, W. E., Bradham, C. A., Brenner, D. A., and Herman, B. 1998. The mitochondrial permeability transition in cell death: a common mechanism in necrosis, apoptosis and autophagy. Biochim. Biophys. Acta 1366:177–196.
Kristal, B. S. and Dubinsky, J. M. 1997. Mitochondrial permeability transition in the central system: induction by calcium cycling-dependent and-independent pathways. J. Neurochem. 69:524–538.
Sullivan, P. G., Thompson, M. B., and Scheff, S. W. 2000. Continuous infusion of cyclosporin A post-injury significantly ameliorates cortical damage following traumatic brain injury. Exp. Neurol. 161:631–637.
Norenberg, M. D., Bai, G., and Murthy, Ch. R. K. 2000. The mitochondrial permeability transition in CNS trauma in vitro. J. Neuropath. Exp. Neurol. 59:459.
Ducis, I., Norenberg, L. O., and Norenberg, M. D. 1989. Effect of ammonium chloride on the astrocyte benzodiazepine receptor. Brain Res. 493:362–365.
Schousboe, A., Meier, E., Drejer, J., and Hertz, L. 1989. Preparation of primary cultures of mouse (rat) cerebellar granule cells. Pages 203–206, in Shahar, A., De Vellis, J., Haber, B., and Vernadakis, A. (eds), A Dissection and Tissue Culture Manual of the Nervous System, Alan R. Liss, New York.
Sullivan, H. G., Martinez, J., Becker, D. P., Miller, J. D., Griffith, R., and Wist, A. O. 1976. Fluid-percussion model of mechanical brain injury in the cat. J. Neurosurg. 45:521–534.
Kane, D. J., Sarafian, T. A., Anton, R., Hahn, H., Gralia, E. B., Valentine, J. S., Ord, T., and Bresdesen, D. E. 1993. Bcl-2 inhibition of neural death: Decreased generation of reactive oxygen species. Science. 262:1274–1276.
Ide, T., Tsutsui, H., Kinugawa, S., Utsumi, H., Kang, D., Hattori, N., Uchida, K., Arimura, K., Egashira, K., and Takeshita, A. 1999. Mitochondrial electron transport complex I is a potential source of oxygen free radicals in the failing myocardium. Circ. Res. 85:357–363.
Banati, R. B., Rothe, G., Valet, G., and Kreutzberg, G. W. 1991. Respiratory activity in brain macrophages: a flow cytometric study on cultured rat microglia. Neuropathol. Appl. Neurobiol. 17:223–230.
Hu, S. X., Chao, C. C., Khanna, K. V., Gekker, G., Peterson, P. K., and Molitor, T. W. 1996. Cytokine and free radical production by porcine microglia. Clin. Immunol. Immunopathol. 78:93–96.
Betz, A. L. 1985. Identification of hypoxanthine transport and xanthine oxidase activity in brain capillaries. J. Neurochem. 44:574–579.
Gehrmann, J., Banati, R. B., Wiessner, C., Hossmann, K. A., and Kreutzberg, G. W. 1995. Reactive microglia in cerebral ischemia: an early mediator of tissue damage? Neuropathol. Appl. Neurobiol. 21:277–89.
Smith, M. E., Van der, M. K., and Somera, F. P. 1998. Macrophage and microglial responses to cytokines in vitro: phagocytic activity, proteolytic enzyme release, and free radical production. J. Neurosci. Res. 54:68–78.
Sudo, S., Tanaka, J., Toku, K., Desaki, J., Matsuda, S., Arai, T., Sakanaka, M., and Maeda, N. 1998. Neurons induce the activation of microglial cells in vitro. Exp. Neurol. 154:499–510.
Hirrlinger, J., Gutterer, J. M., Kussmaul, L., Hamprecht, B., and Dringen, R. 2000. Microglial cells in culture express a prominent glutathione system for the defense against reactive oxygen species. Dev. Neurosci. 22(5–6):384–392.
Xiong, Y., Gu, Q., Peterson, P. L., Muizelaar, J. P., and Lee, C. P. 1997. Mitochondrial dysfunction and calcium perturbation induced by traumatic brain injury. J. Neurotrauma. 14:23–34.
Xiong, Y., Peterson, P. L., Verweij, B. H., Vinas, F. C., Muizelaar, J. P., and Lee, C. P. 1998. Mitochondrial dysfunction after experimental traumatic brain injury: combined efficacy of SNX-111 and U-101033E. J. Neurotrauma. 15:531–544.
Szabo, I. and Zoratti, M. 1991. The giant channel of the inner mitochondrial membrane is inhibited by cyclosporin A. J. Biol. Chem. 266:3376–3379.
Fruman, D. A., Klee, C. B., Bierer, B. E., and Burakoff, S. J. 1992. Calcineurin phosphatase activity in T lymphocytes is inhibited by FK 506 and cyclosporin A. Proc. Natl. Acad. Sci. 89:3686–3690.
Friberg, H., Ferrand-Drake, M., Bengtsson, F., Halestrap, A. P., and Wieloch, T. 1998. Cyclosporin A, but not FK 506, protects mitochondria and neurons against hypoglycemic damage and implicates the mitochondrial permeability transition in cell death. J. Neurosci. 18:5151–5159.
Zamzami, N., Hirsch, T., Dallaporta, B., Petit, P. X., and Kroemer, G. 1997. Mitochondrial implication in accidental and programmed cell death: apoptosis and necrosis. J. Bioenerg. Biomembr. 29:185–193.
Zoratti, M. and Szabo, I. 1994. Electrophysiology of the inner mitochondrial membrane. J. Bioenerg. Biomembr. 26:543–553.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Panickar, K.S., Jayakumar, A.R. & Norenberg, M.D. Differential Response of Neural Cells to Trauma-Induced Free Radical Production In Vitro. Neurochem Res 27, 161–166 (2002). https://doi.org/10.1023/A:1014875210852
Issue Date:
DOI: https://doi.org/10.1023/A:1014875210852