Abstract
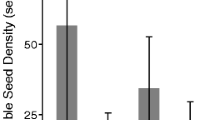
Populus nigra L. var betulifolia and Salix alba L. var alba are early successional riparian tree species threatened throughout Continental Europe by significant changes to the natural physical processes governing their natural habitat – geomorphologically active floodplains. River management activities have dramatically altered natural patterns of river flow and rates of sediment delivery along rivers, with possible consequences for the balance between sexual and asexual regeneration strategies in these species. Conservation strategies will benefit from a greater understanding of the ways in which dynamic physical processes on the floodplain influence sexual and asexual recruitment. This paper describes a field survey investigating the relative abundance and spatial distributions of P. nigra and S. alba sexual and asexual recruits during the first years of establishment along a braided gravel bed river. Sexual and asexual recruits were identified by excavation along transects in a wet and a dry field season and distributional differences were described in terms of elevation on the floodplain, local sediment type and exposure to floodwaters. Regeneration was overwhelmingly from seed in the first 2–3 years following recruitment, but poor survival rates among sexual recruits saw a shift in the relative abundance of regeneration strategies over time. In relating hydrological data to recruitment, unseasonal flood disturbances had a negative effect on recruitment from seed and a positive effect on vegetative regeneration. Seedlings were associated with fine sediment deposits and were restricted primarily to low elevations on the flood plain, while asexual recruits had a wider spatial distribution. Certain microsite types were unique to either regeneration strategy.
Similar content being viewed by others
References
Arens, P., Coops, H., Jansen, J. and Vosman, B. (1998) Molecular genetic analysis of black poplar (Populus nigra L.) along Dutch rivers. Mol. Ecol. 7, 11-18.
Barsoum, N. (1998) 'A Comparison of Vegetative and Non-vegetative Regeneration Strategies in Populus nigra and Salix alba', Ph.D. Thesis, Department of Geography, University of Cambridge. 369 pp.
Barsoum, N. and Hughes, F.M.R. (1998) Regeneration response of black poplar to changing river levels. In H. Wheater and C. Kirkby (eds) Hydrology in a Changing Environment, Volume 1. John Wiley & Sons, Chichester, pp. 397-412.
Beismann, H., Barker, J.H.A., Karp, A. and Speck, T. (1997) AFLP analysis sheds light on distribution of two Salix species and their hybrids along a natural gradient. Mol. Ecol. 6, 989-993.
Bloom, A.J., Chapin III, F.S. and Mooney, H.A. (1985) Resource limitations in plants -- an economic analogy. Ann. Rev. Ecol. Syst. 16, 363-392.
Bond, W.J. and Midgley, J.J. (2001) Ecology of sprouting in woody plants: the persistence niche. Trends Ecol. Evol. 16(1), 45-51.
Bradley, C.E. and Smith, D.G. (1986) Plains cottonwood recruitment and survival on a prairie meandering river floodplain, Milk River, southern Alberta and northern Montana. Can. J. Bot. 64, 1433-1442.
Caraco, T. and Kelly, C.K. (1991) On the adaptive value of physiological integration in clonal plants. Ecology 72(1), 81-93.
Carlson, M.C. (1938) Nodal adventitious roots in willow stems of different ages. Am. J. Bot. 37, 555-561.
Carlson, M.C. (1950) The formation of nodal adventitious roots in Salix cordata. Am. J. Bot. 25, 721-725.
Cheliak, W.M. and Pitel, J.A. (1984a) Electrophoretic identification of clones in trembling aspen. Can. J. For. Res. 14, 740-743.
Cook, R.E. (1979) Asexual reproduction: a further consideration. Am. Nat. 113, 769-772.
Cook, R.E. (1985) Growth and development in clonal plant populations. In J.B.C. Jackson, L.W. Buss and R.E. Cook (eds) Population Biology and Evolution of Clonal Organisms. Yale University Press, New Haven, Connecticut, USA, pp. 399-436.
De Steven, D. (1989) Genet and ramet demography of Oenocarpus mapora ssp. mapora, A clonal palm of panamanian tropical moist forest. J. Ecol. 77, 579-596.
Ellstrand, N.C. and Antonovics, J. (1985) Experimental studies of the evolutionary significance of sexual reproduction II. A test of the density-dependent selection hypothesis. Evolution 39(3), 657-666.
Eriksson, O. (1989) Seedling dynamics and life histories in clonal plants. Oikos 55, 231-238.
Eriksson, O. (1992) Evolution of seed dispersal and recruitment in clonal plants. Oikos, 63, 439-448.
Erôme, G. (1982) 'Contribution à la Connaissance Ecoécologique du Castor (Castor fiber) dans la Vallée du Rhône'. Ph.D. Thesis, Claude-Bernard University, Lyon, France.
Escaravage, N., Questiau, S., Pornon, A., Doche, B. and Taberlet, P. (1998) Clonal diversity in a Rhododendron ferrugineum L. (Ericaceae) population inferred from AFLP markers. Mol. Ecol. 7, 975-982.
Everitt, B.L. (1968) Use of the cottonwood in an investigation of the recent history of a floodplain. Am. J. Sci. 266, 417-439.
Falinska, K. (1995) Genet disintegration in Filipendula ulmaria: Consequences for population dynamics and vegetation succession. J. Ecol. 83, 9-21.
Gardner, S.N. and Mangel, M. (1999) Modeling investments in seeds, clonal offspring and translocation in a clonal plant. Ecology 80(4), 1202-1220.
Gom, L.A. and Rood, S.B. (1999) Patterns of clonal occurrence in a mature cottonwood grove along the Oldman River, Alberta. Can. J. Bot. 77, 1095-1105.
Harada, Y., Kawano, S. and Iwasa, Y. (1997) Probability of clonal identity: inferring the relative success of sexual versus clonal reproduction from spatial genetic patterns. J. Ecol. 85, 591-600.
Held, M.E. (1983) Pattern of beech regeneration in the cast-central United States. Bull. Torr Bot. Club 110, 55-62.
Hermandutz, L.A., Innes, D.J. and Weis, I.M. (1989) Clonal structure of arctic dwarf birch (Betula glandulosa) at its northern limit. Am. J. Bot. 76, 755-761.
Houle, G. and Babeux, P. (1993) Temporal variations in the rooting ability of cuttings of Populus balsamifera and Salix planifolia from natural clone populations of subarctic Quebec. Can. J. For. Res. 23, 2603-2608.
Huenneke, L.A. (1985) Spatial distribution in thickets of Alnus incana ssp. rugosa a clonal shrub. Am. J. Bot. 72(1), 152-158.
Hunter, C.L. (1993) Genotypic variation and clonal structure in coral populations with different disturbance histories. Evolution 47(4), 1213-1228.
Jelinski, D.E. and Cheliak, W.M. (1992) Genetic diversity and spatial subdivision of Populus tremuloides in a heterogeneous landscape. Am. J. Bot. 79(7), 728-736.
Johansson, M.E. and Nilsson, C. (1993) Hydrochory, population dynamics and distribution of the clonal aquatic plant Ranunculus lingua. J. Ecol. 81, 81-91.
Johnson, C.A. (1994) Woodland expansion in the Platte River, Nebraska: patterns and causes. Ecol. Mongr. 64, 45-84.
Kudoh, H., Shibaike, H., Wigham, D.F. and Kawano, S. (1999) Genet structure and determinants of clonal structure in a temperate deciduous woodland herb, Uvularia perfoliata. J. Ecol. 87, 244-257.
Legionnet, A., Faivre-Rampant, P., Villar, M. and Lefèvre, F. (1997) Sexual and asexual reproduction in natural stands of Populus nigra. Bot. Acta 110, 257-263.
Lovett-Doust, L. (1981) Population dynamics and local specialisation in a clonal perennial (Ranunculus repens). I. The dynamics of ramets in contrasting habitats. J. Ecol. 69, 743-755.
Lynch, M. (1996) A quantitative-genetic perspective on conservation issues. In J.C. Avise and J.L. Hamrick (eds) Conservation Genetics Case Studies from Nature. Chapman and Hall, New York, USA.
Maddox, G.D., Cook, R.E., Wimberger, P.H. and Gardescu, S. (1989) Clonal structure in four Solidago allissima (Asteraceae) Populations: rhizome connections within genotypes. American Journal of Botany 76, 318-326.
Mandujano, M.C., Montana, C., Méndez, I. and Golubov, J. (1998) The relative contributions of sexual reproduction and clonal propagation in Opuntia rastrera from two habitats in the Chihuahuan Desert. J. Ecol. 86, 911-921.
McBride, J.R. and Strahan, J. (1984) Establishment and survival of woody riparian species on gravel bars of an intermittent stream. Am. Midl. Nat., 112, 235-245.
McKay, S.J. (1996) 'The Impact of River Regulation on Establishment Processes of Riparian Black Cottonwood', Masters Thesis, College of Forest Resources, University of Washington.
Michaels, H.J. and Bazzaz, F.A. (1989) Individual and population responses of sexual and apomictic plants to environmental gradients. Am. Nat., 134, 190-207.
Noble, M.G. (1979) The origin of Populus deltoides and Salix interior zones on point bars along the Minnesota River. Am. Midl. Nat. 102, 59-67.
Parker, K.C. and Hamrick, J.L. (1992) Genetic diversity and clonal structure in a columnar cactus, Lophocereus schottii. Am. J. Bot. 79(1), 86-96.
Petts, G.E. (1989) Historical analysis of fluvial hydrosystems. In G.E. Petts (ed.) Historical Change of Large Alluvial Rivers: Western Europe. John Wiley & Sons Ltd., Chichester, pp. 1-18.
Phillips, D.L. and Shure, D.J. (1990) Patch-size effects on early succession in southern Appalachian forests. Ecology 71, 204-212.
Piquot, Y., Petit, D., Valero, M., Cuguen, J., de Laguerie, P. and Vernet, P. (1998) Variation in sexual and asexual reproduction among young and old populations of the perennial macrophyte Sparganium erectum. Oikos 82, 139-148.
Pitelka, L.F. and Ashmun, J.W. (1985) Physiology and integration of ramets in clonal plants. In J.B.C. Jackson, L.W. Buss and R.E. Cook (eds) Population Biology and Evolution of Clonal Organisms. Yale University Press, New Haven, Connecticut, USA, pp. 399-436.
Prati, D. and Schmid, B. (2000) Genetic differentiation of life-history traits within populations of the clonal plant Ranunculus reptans. Oikos 90, 442-456.
Read, J. and Hill, R.S. (1985) Dynamics of Nothofagus-dominated rainforest on mainland Australia and lowland Tasmania. Vegetatio 63, 67-78.
Rood, S.B., Hillman, C., Sanche, T. and Mahoney, J.M. (1994) Clonal reproduction of riparian cottonwoods in southern Alberta. Can. J. Bot. 72, 1766-1774.
Sacchi, C.F. and Price, P.W. (1992) The relative roles of biotic and abiotic factors in seedling demography of arrayo willow (Salix lasiolepsis: Salicaceae) Am. J. Bot. 79, 395-405.
Schier, G.A. and Campbell, R.B. (1976) Differences among Populus species in ability to form adventitious shoots and roots. Can. J. For. Res. 6, 253-261.
Shafroth, P.B., Scott, M.L. and Friedman, J.M. (1994) Establishment, sex structure and breeding system of an exotic riparian willow (Salix × rubens) Am. Midl. Nat. 132, 159-172.
Tardif, J. and Bergeron, Y. (1999) Population dynamics of Fraxinus nigra in response to flood-level variations in Northwestern Quebec. Ecol. Mongr. 69(1), 107-125.
Van Splunder, I., Coops, H., Voesenek, L.A.C.J. and Blom, C.W.P.M. (1995) Establishment of alluvial forest species in floodplains: the role of dispersal timing, germination characteristics and water level fluctuations. Acta Bot. Neerl. 44(3), 269-278.
Van Splunder, I., Voesenek, L.A.C.J., Coops, H., De Vries, X.J.A. and Blom, C.W.P.M. (1996) Morphological responses of seedlings of four species of Salicaceae. Can. J. Bot. 74, 1988-1995.
Zasada, J.C., Viereck, L.A., Foote, M.J., Parkenson, R.H., Wolff, J.O. and Lankford, L.A. (1981) Natural regeneration of balsam poplar following harvesting in the Susitna Valley, Alaska For. Chron. 57, 57-65.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Barsoum, N. Relative contributions of sexual and asexual regeneration strategies in Populus nigra and Salix alba during the first years of establishment on a braided gravel bed river. Evolutionary Ecology 15, 255–279 (2001). https://doi.org/10.1023/A:1016028730129
Issue Date:
DOI: https://doi.org/10.1023/A:1016028730129