Abstract
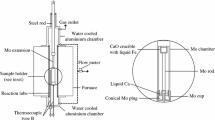
The Knudsen method of mass-spectrometry and the integral option of the Knudsen effusion method realized under conditions of ultrahigh oilless vacuum are used in the overall temperature range from 1440 to 1916 K to investigate the evaporation of pure iron and copper and of a dilute liquid Fe–Cu solution with the copper content ranging from 0.17 to 10.1 at. %. The obtained data are used to calculate the values of standard sublimation enthalpy for Fe and Cu, as well as the partial and integral thermodynamic characteristics of liquid alloys of iron with copper. Significant positive deviations from Raoult's law are observed.
Similar content being viewed by others
REFERENCES
Choudary U.V., Serkin J.A., and Belton G.R., Metall. Trans. B, 1975, vol. 6, p. 399.
Chipman, J., Discuss. Faraday Soc., 1948, no. 4, p. 3.
Zaitsev, A.I., Korolev, N.V., and Mogutnov, B.M., Teplofiz. Vys. Temp., 1989, vol. 27, no. 3, p. 465.
Zaitsev, A.I., Korolyov, N.V., and Mogutnov, B.M., High Temp. Sci., 1990, vol. 28, p. 341.
Zaitsev, A.I., Zemchenko, M.A., and Mogutnov, B.M., Zh. Fiz. Khim., 1990, vol. 64, no. 5, p. 1187.
Zaitsev, A.I., Priselkov, Yu.A., and Nesmeyanov, An.N., Teplofiz. Vys. Temp., 1982, vol. 20, no. 4, p. 781.
Iszkowsski, R.P., Margrave, J.L., and Robinson, S.M., J. Phys. Chem., 1963, vol. 64, no. 2, p. 229.
Gurvich, L.V., Vestn. Akad. Nauk SSSR, 1983, no. 3, p. 54.
Sidorov, L.N., Korobov, M.V., and Zhuravleva, L.V., Mass-spektral'nye termodinamicheskie issledovaniya (Mass-Spectral Thermodynamic Investigations), Moscow: Mosk. Gos. Univ. (Moscow State Univ.), 1985.
Mann, J.B., Recent Development in Mass-Spectrometry, Proc. Int. Conf. on Mass Spectroscopy, Ogata, T. and Hayakawa, T., Eds., Tokyo: Univ. Park Press, 1970, p. 814.
Kornilov, A.N., Stepina, L.B., and Sokolova, V.A., Zh. Fiz. Khim., 1972, vol. 42, no. 11, p. 2975.
Zaitsev, A.I., Zemchenko, M.A., and Mogutnov, B.M., Zh. Fiz. Khim., 1990, vol. 64, no. 12, p. 3377.
Belton, J.R. and Fruehan, R.J., J. Phys. Chem., 1967, vol. 71, p. 1402.
Morris, J.P. and Zellars, G.R., J. Met. Trans. Metall. Soc. AIME, 1956, vol. 8, p. 1086.
Koros, P.J. and Chipman, J., J. Met. Trans. Metall. Soc. AIME, 1956, vol. 8, p. 1102.
Oelsen, W., Schurmann, E., and Florin, C., Arch. Eisen-huettenwes., 1961, vol. 32, p. 719.
Woolley, F. and Elliott, J.F., Trans. Metall. Soc. AIME, 1967, vol. 239, p. 1872.
El-Hasan, A., Abdel-Aziz, K., Vertman, A.A., and Samarin, A.M., Izv. Akad. Nauk SSSR Met., 1966, no. 3, p. 19.
Langenberg, F.C., J. Met. Trans. Metall. Soc. AIME, 1956, vol. 8, p. 1024.
Hultgren, R., Desai, P.D., Hawkins, D.T., et al., Selected Values of the Thermodynamic Properties of Binary Alloys, Metals Park: American Society of Metals, 1973.
Lupis, C.H.P., Khimicheskaya termodinamika materia-lov (Chemical Thermodynamics of Materials), Moscow: Metallurgiya, 1989 (Russ. transl.).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zaitsev, A.I., Shelkova, N.E., Litvina, A.D. et al. An Investigation of Evaporation of Liquid Alloys of Iron with Copper. High Temperature 39, 388–394 (2001). https://doi.org/10.1023/A:1017554408124
Issue Date:
DOI: https://doi.org/10.1023/A:1017554408124