Abstract
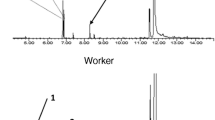
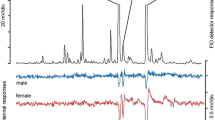
The weevil Oxyops vitiosa is an Australian species imported to Florida, USA, for the biological control of the invasive weed species Melaleuca quinquenervia. Larvae of this species feed on leaves of their host and produce a shiny orange secretion that covers the integument. When this secretion is applied at physiological concentrations to dog food bait, fire ant consumption and visitation are significantly reduced. Gas chromatographic analysis indicates that the larval secretion qualitatively and quantitatively resembles the terpenoid composition of the host foliage. When the combination of 10 major terpenoids from the O. vitiosa secretion was applied to dog food bait, fire ant consumption and visitation were reduced. When these 10 terpenoids were tested individually, the sesquiterpene viridiflorol was the most active component in decreasing fire ant consumption. Fire ant visitation was initially (15 min after initiation of the study) decreased for dog food bait treated with viridiflorol and the monoterpenes 1,8-cineole and α-terpineol. Fire ants continued to avoid the bait treated with viridiflorol at 18 μg/mg dog food for up to 6 hr after the initiation of the experiment. Moreover, ants avoided bait treated with 1.8 μg/mg for up to 3 hr. The concentrations of viridiflorol, 1,8-cineole, and α-terpineol in larval washes were about twice that of the host foliage, suggesting that the larvae sequester these plant-derived compounds for defense against generalist predators.
Similar content being viewed by others
REFERENCES
Aplin, R. T., Benn, M. H., and Rothschild, M. 1968. Poisonous alkaloids in the body tissues of the Cinnabar Moth (Callimorpha jacobaeae L.). Nature 219:747–748.
Balciunas, J. K. and Buckingham, G. R. 1996. lease of the Australian weevil Oxyops vitiosa for control of melaleuca, Melaleuca quinquenervia. Unpublished report submitted to TAG form Release of Biological Control Agent, 23 pp.
Balciunas, J. K., Burrows, D. W., and Purcell, M. F. 1994. Field and laboratory host ranges of the Australian weevil, Oxyops vitiosa (Coleoptera: Curculionidae), a potential biological control agent for the paperbark tree, Melaleuca quinquenervia. Biol. Control 4:351–360.
Blum, M. S., Wallace, J. B., Duffield, R. M., Brand, J. M., FÁles, H. M., and Sokoloski, E. A. 1978. Chrysomelidial in the defensive secretion of the leaf beetle Gastrophysa cyanea Melsheimer.J. Chem. Ecol. 4:47–53.
Bowers, M. D. 1990. Recycling plant natural products for insect defense, pp. 353–386, in D. L. Evans and J. O. Schmidt (eds.). Insect Defenses: Adaptive Mechanisms and Strategies of Prey and Predators. State University of New York Press, Albany, New York.
Bowers, M. D. and Collinge, S. K. 1992. Fate of iridoid glycosides in different life stages of the buckeye, Junonia coenia (Lepidoptera: Nymphalidae). J. Chem. Ecol. 18:817–831.
Bowers, M. D., Boockvar, K., and Collinge, S.K. 1993. Iridoid glycosides of Chelone glabra (Scrophulariaceae) and their sequestration by larvae of a sawfly, Tenthredo grandis (Tenthredinidae). J. Chem. Ecol. 19:815–823.
Brophy, J. J., Boland, D. J., and Lassak, E. V. 1989. Leaf essential oils of Melaleuca and Leptospermum species from tropical Australia, pp. 193–203, in D. J. Boland (ed.). Trees for the Tropics, Growing Australian Multipurpose Trees and Shrubs in Developing Countries. Australian Centre for International Agricultural Research, Canberra, Australia.
Center, T. D., Van, T. K., Rayachhetry, M., Buckingham, G. R., Dray, F. A., Wineriter, S., Purcell, M. F., and Pratt, P. D. 2000. Field colonization of the Melaleuca snout beetle (Oxyops vitiosa) in south Florida. Biol. Control 19: 112–123.
Ciomperlik, M. A., Chandler, J. M., and Deloach, C. J. 1992. Predation by red imported fire ant on Tyta luctuosa released for control of field bindweed. Southwest. Entomol. 17:267–269.
Cornelius, M. L. and Bernays, E. A. 1995. The effect of plant chemistry on the acceptability of caterpillar prey to the Argentine ant lridomyrmex humilils (Hymenoptera: Formicidae). J. Insect Behav. 8:579–593.
Crawley, M. J. 1989. The successes and failures of weed biocontrol using insects. Biocontrol News Info. 19:213–223.
Debbrecht, F. J. 1985. Qualitative and quantitative analysis by gas chromatography, pp. 359–421, in R. L. Grob (ed.). Modern Practices of Gas Chromatography. Wiley, New York.
Degen, T. and Stadler, E. 1998. Oviposition of carrot fly (Psila rosae) in response to foliage and leaf surface extracts of the host plants. Chemoecology 8:39–49. 314 WHEELER, MASSEY, AND SOUTHWELL
Eisner, T., Ziegler, R., Mccormick, J. L., Eisner, M., Hoebeke, E. R., and Meinwald, J. 1994. Defensive use of an acquired substance (carminic acid) by predaceous insect larvae. Experientia 50:610–615.
Elvin, M. K., Stimac, J. L., and Whitcomb, W. H. 1983. Estimating rates of arthropod predation on velvetbean caterpillar larvae in soybeans. Fla. Entomol. 66:319–330.
Espelie, K. E. and Bernays, E. A. 1989. Diet-related differences in the cuticular lipids of Manduca sexta larvae. J. Chem. Ecol. 15:2003–2017.
Goeden, R. D. and Louda, S. M. 1976. Biotic interference with insects imported for weed control.Annu. Rev. Entomol. 21:325–342.
Gomez, N. E., Witte, L., and Hartmann, T. 1999. Chemical defense in larval tortoise beetles: Essential oil composition of fecal shields of Eurypedus nigrosignata and foliage of its host plant, Cordia curassavica. J. Chem. Ecol. 25:1007–1027.
Howard, J. J., Cazin, J., Jr., and Wiemer, D. F. 1988. Toxicity of terpenoid deterrents to the leafcutting ant Atta cephalotes and its mutualistic fungus. J. Chem. Ecol. 14:59–69.
Howard, J. J., Green, T. P., and Wiemer, D. F. 1989. Comparative deterrency of two terpenoids to two genera of attine ants. J. Chem. Ecol. 15:2279–2288.
Hubbell, S. P., Howard, J. J., and Wiemer, D. F. 1984. Chemical leaf repellency to an attine ant: Seasonal distribution among potential host plant species. Ecology 65: 1067–1076.
Ireland, B. F. 1999. Essential oils of Eucalyptus miniata and Melaleuca quinquenervia. Honors thesis.School of Chemistry, University of New South Wales, Sydney, 55 pp.
Jeffries, M. J. and Lawton, J.H. 1984. Enemy free space and the structure of ecological communities Biol. J. Linn. Soc. 23:269–289.
Julien, M. H. and Griffiths, M.W. 1998. Biological Control ofWeeds, aWorld Catalogue of Agents and their Target Weeds, 4th ed., CAB International, Oxon, United Kingdom.
Kharboutli, M.W. and Mack, T. P. 1991. Relative and seasonal abundance of predaceous arthropods in Alabama peanut fields as indexed by pitfall traps. J. Econ. Entomol. 84:1015–1023.
Lofgren, C. S., Banks, W. A., and Glancey, B. M. 1975. Biology and control of imported fire ants. Annu. Rev. Entomol. 20:1–30.
Montgomery, B. R. and Wheeler, G. S. 2000. Anti-predatory activity of the weevil Oxyops vitiosa: A biological control agent of Melaleuca quinquenervia. J. Insect Behav. 13:915–926.
Ohmart, C. P. and Larsson, S. 1989. Evidence for absorption of eucalyptus essential oils by Paropsis atomaria Oliver (Coleoptera: Chrysomelidae). J. Aust. Entomol. Soc. 28:201–205.
Olmstead, K. L. and Denno, R. F. 1993. Effectiveness of tortoise beetle larval shields against different predator species. Ecology 74:1394–1405.
Pavis, C., Malosse, C., Ducrot, P. H., Howse, F., Jaffe, K., and Dscoins, C. 1992. Defensive secretion of first-instar larvae of rootstalk borer weevil, Diaprepes abbreviatus L. (Coleoptera: Curculionidae), to the fire-ant Solenopsis geminata (F.) (Hymenoptera: Formicidae). J. Chem. Ecol. 18:2055–2068.
Purcell, M. F. and Balciunas, J. K. 1994. Life history and distribution of the Australian weevil Oxyops vitiosa (Coleoptera: Curculionidae), a potential biological control agent for Melaleuca quinquenervia (Myrtaceae). Ann. Entomol. Soc. Am. 87:867–873.
Ramanoelina, P. A. R., Viano J., Bianchini, J. P., and Gaydou, E. M. 1994. Occurrence of various chemotypes in Niaouli (Melaleuca quinquenervia) essential oils from Madagascar using multivariate statistical analysis. J. Agric. Food Chem. 42:1177–1182.
Rees, C. J. C. 1969. Chemoreceptor specificity associated with choice of feeding site by the beetle Chrysolina brunsvicensis on its foodplant Hypericum hirsutum. Entomol. Exp. Appl. 12:565–583.
SAS Institute. 1990. SAS/STAT User's Guide, Version 6. SAS Institute, Cary, North Carolina.
Shorey, H. H., Gaston, K. J., Gerber, R. G., Phillips, P. A., and Wood, D. L. 1992. Disruption of foraging by Argentine ants, Iridomyrmex humilis (Mayer) (Hymenoptera: Formicidae), in WEEVIL DEFENSE SEQUESTERED FROM HOST 315 citrus trees through the use of semiochemicals and related chemicals. J. Chem. Ecol. 18: 2131–2142.
Shorey, H. H., Gaston, L. K., Gerber, R. G., Sisk, C. B., and Phillips, P. A. 1996. Formulating farnesol and other ant-repellent semiochemicals for exclusion of Argentine ants (Hymenoptera: Formicidae) from citrus trees. Environ. Entomol. 25: 114–119.
Slansky, F., Jr. and Scriber, J. M. 1985. Food consumption and utilization, pp. 87–163, in G. A. Kerkut and L. I. Gilbert (eds.). Comprehensive Insect Physiology, Biochemistry and Pharmacology, Vol. 4, Pergamon, Oxford.
Southwell, I. A., Maddox, C. D. A., and Zalucki, M. P. 1995. Metabolism of 1,8-cineole in tea tree (Melaleuca alternifolia and M. linariifolia) by pyrgo beetle (Paropsisterna tigrina). J. Chem. Ecol. 21:439–453.
Wink, M., Hartmann, T., Witte, L., and Rheinheimer, J. 1982. Interrelationship between quinolizidine alkaloid producing legumes and infesting insects: Exploitation of the alkaloid-containing phloem sap of Cytisus scoparius by the broom aphid Aphis cytisorum. Z. Naturforsch. 37C:1081–1086.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wheeler, G.S., Massey, L.M. & Southwell, I.A. Antipredator Defense of Biological Control Agent Oxyops vitiosa Is Mediated by Plant Volatiles Sequestered from the Host Plant Melaleuca quinquenervia. J Chem Ecol 28, 297–315 (2002). https://doi.org/10.1023/A:1017982007812
Issue Date:
DOI: https://doi.org/10.1023/A:1017982007812