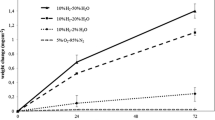
Abstract
The oxidation of type 304L stainless steel wasinvestigated at 873 K in the presence of O2and O2 + 10% H2O. Oxidation timevaried between 1 and 672 hr. The oxidized samples wereinvestigated by a number of surface-analytical techniques includinggrazing-angle XRD, SEM/EDX, auger spectroscopy, SIMS andXPS. Oxidation in dry oxygen results in the formation acorundum-type oxide (Me2O3) withadditional formation of spinel oxides after prolonged exposure. Theoxide layer contained mainly chromium, with smalleramounts of Fe and Mn. Oxidation in the presence of watervapor results in an oxide that contains more Fe and less Cr, the outer part of the oxide beingdepleted in Cr. In the presence of water vapor, a massloss is detected after prolonged exposure. We show thatthe mass loss is caused by chromium evaporation. The volatile species is suggested to beCrO2(OH)2.
Similar content being viewed by others
REFERENCES
A. Rahmel and J. Tobolski,Corros. Sci. 5, 333 (1965).
C. T. Fujiiand R. A. Meussner, J. Electrochem. Soc. 111, 1215 (1964).
M. Thiele, H. Teichmann, W. Schwarz, and W. J. Quadakkers, VGB Kraftwerkste. 2, 77 (1997).
P. Kofstad, High-TemperatureCorrosion, Chap. 11 (Elsevier Applied Science, London, 1988), p. 382.
I. Kvernes, M. Oliveira, and P. Kofstad, Corros. Sci. 17, 2371 (1977).
A. S. Khanna and P. Kofstad, Intern. Conf. Microsc. Oxid. (Institute Of Materials, London, 1990), p. 113.
F. Armanet, A. Vejux, and G. Beranger, Behaviouirof High-Temperature Alloys in Agressive Environments, EUR 6814 (The Metals Society, London), p. 423.
S. Jianian, Z. Longjiang, and L. Tiefan, Oxid. Met. 48, 347 (1997).
T. Norby, J. Phys. 3,99 (1993).
C. S. Tedmond, Jr., J.Electrochem. Soc. 113, 766 (1966).
H. C. Graham and H. H. Davies, J. Am. Ceram. Soc. 54, 89 (1971).
C. A. Stearns, F. J. Kohl, and G.C. Fryburg, J. Electrochem. Soc. 121, 945 (1974).
B. B. Ebbinghaus, Combustion Flame 93, 119 (1993).
P. E. Wretblad, Z.Anorg. Chem. 189, 331 (1930).
Rights and permissions
About this article
Cite this article
Asteman, H., Svensson, JE., Johansson, LG. et al. Indication of Chromium Oxide Hydroxide Evaporation During Oxidation of 304L at 873 K in the Presence of 10% Water Vapor. Oxidation of Metals 52, 95–111 (1999). https://doi.org/10.1023/A:1018875024306
Issue Date:
DOI: https://doi.org/10.1023/A:1018875024306