Abstract
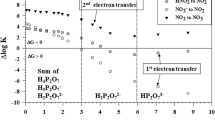
Manganese oxides are strong environmental oxidants recently found to be involvedin the nitrogen cycle. Of the several possible reactions with reduced nitrogen species,the reduction of MnO2 by nitrite has only received marginal attention. Yet, this reaction might explain why nitrification can occur in the absence of O2, observed in both sediments and water columns. We have determined the stoichiometry of this reaction, as well as the chemical kinetics and the activation parameters, using a soluble polymeric form of MnO2. The reaction rate decreases with increasing pH and decreasing temperature. The reaction is first order in each reactant with a second order rate constant (k) = 493 M-1 min-1 at 21.5 °C and pH = 5.00. The energy of activation (Ea = 9.370 kJ/mole) and the entropy of activation (Δ S‡ = -169.5 J/mole) show the reaction to be associative and diffusion controlled, occurring via an inner-sphere mechanism, likely with O atom transfer from MnO2 to HNO2. The reaction is proton assisted and slowsdown at pH ≥ 5.5 where NO2 - and MnO2 (unprotonated and negatively charged) become the dominant species. In natural waters and sediments where anaerobic nitrification has been observed the pH is higher than this. Thus, the thermodynamically favorable reaction will likely proceed by microbial mediation.
Similar content being viewed by others
References
Atwood J. D. (1985) Inorganic and Organometallic Reaction Mechanisms. Brooks/Cole Publishing Company. Monterey, CA.
Bartlett R. J. (1981) Nonmicrobial nitrite to nitrate transformation in soils. J. Amer. Soil Sci. Soc. 45, 1054–1058.
Connors K. A. (1990) Chemical Kinetics: The Study of Reaction Rates in Solution. VCH Publishers, Inc., New York.
Cotton F. A., Wilkinson G., Murillo C. A. and Bochmann M. (1999) Advanced Inorganic Chemistry (6th ed.). John Wiley & Sons, Inc., New York.
Froelich P. N., Klinkhammer G. P., Bender M. L., Luedtke N. A., Heath G. R., Cullen D., Dauphin P., Hammond D., Hartman B. and V. Maynard V. (1979) Early oxidation of organic matter in pelagic sediments of the eastern equatorial Atlantic: suboxic diagensis. Geochim. Cosmochim. Acta. 43, 1075–1090.
Hulth S., Aller R. C. and Gilber F. (1999) Coupled anoxic nitrification/manganese reduction in marine sediments. Geochim. Cosmochim. Acta. 63(1), 46–66.
Hulthe G., Hulth S. and Hall, P. O. J. (1998) Effect of oxygen on degradation rate of refractory and labile organic matter in continental margin sediments. Geochim. Cosmochim. Acta. 62(8), 1319–1328.
Kaplan W. A. (1983) Nitrification (Ch. 5). In Nitrogen in the Marine Environment. (eds. E. J. Carpenter and D. G. Capone), pp. 139–190. Academic Press, New York.
Kostka J. E., Luther III G. W. and Nealson K. H. (1995) Chemical and biological reduction of Mn(III)-pyrophosphate complexes: potential importance of dissolved Mn(III) as an environmental oxidant. Geochim. Cosmochim. Acta. 59(5), 885–894.
Laha S. and Luthy R. G. (1990) Oxidation of aniline and other primary aromatic amines by manganese dioxide. Envirol Sci. Technol. 24, 363–373.
Libes S. M. (1992) An Introduction to Marine Biogeochemistry. John Wiley & Sons, Inc.
Luther III G. W., Ruppel D. T. and Burkhard C. (1999a) Reactivity of dissolved Mn(III) complexes and Mn(IV) species with reductants: Mn redox chemistry without a dissolution step? In Mineral-Water Interfacial Reactions: Kinetics and Mechanisms. (eds. D. L. Sparks and T. J. Grundl), pp. 265–280. American Chemical Society.
Luther III G. W., Theberge S. M. and Rickard D. T. (1999b) Evidence for aqueous clusters as intermediates during zinc sulfide formation. Geochim. Cosmochim. Acta 63, 3159–3169.
Luther III G. W., Sundby B., Lewis R. L., Brendel P. J., and Silverberg N. (1997) Interactions of manganese with the nitrogen cycle: alternative pathways to dinitrogen. Geochim. Cosmochim. Acta. 61, 4043–4052.
Luther III G. W., Nuzzio D. and Wu J. (1994) Speciation of manganese in Chesapeake Bay waters by voltammetric methods. Anal. Chim. Acta 284, 473–480.
Luther III G. W., Church T. M. and Powell D. (1991) Sulfur speciation and sulfide oxidation in the water column of the Black Sea. Deep-Sea Research 38(S2), S1121–S1127.
Luther III G. W. (1990) The frontier molecular orbital theory approach in geochemical processes. In Aquatic Chemical Kinetics (ed. W. Stumm), Chap. 6, pp. 173–198. John Wiley & Sons, New York.
McArdell C. S., Stone A. T. and Tian J. (1998) Reactions of EDTA and related aminocarboxylate chelating agents with CoIIIOOH (Heterogenite) and MnIIIOOH (Manganite). Environ. Sci. Technol. 32, 2923–2930.
Millero F. J., Hubinger S., Fernandez M. and Garnett S. (1987) Oxidation of H2S in seawater as a function of temperature, pH, and ionic strength. Environ. Sci. Technol. 21, 439–443.
Miyajima T. (1994). Mud-water fluxes of inorganic nitrogen and manganese in the pelagic region of Lake Biwa: Seasonal dynamics and impact on the hypolimnetic metabolism. Archivfur Hydrobiologie 130(3), 303–324.
Morel F. M. M. and Hering J. G. (1993) Principles and Applications of Aquatic Chemistry. John Wiley & Sons, Inc., New York.
Morse J.W. and Casey W. H. (1988) Ostwald processes and mineral paragenesis in sediments. Amer. J. Sci. 288, 537–560.
Mortimer R. J. G., Davey J. T., Krom M. D., Watson P. G., Frickers P. E. and Clifton R. J. (1999) The effect of macrofauna on porewater profiles and nutrient fluxes in the intertidal zone of the Humber Estuary. Est. Coast. Shelf Sci. 48, 683–699.
Mortimer R., Krom M. D., Hall P. O. J., Hulth S. and Stahl H. (1998) Use of gel probes for the determination of high resolution solute distributions in marine and estuarine pore waters. Mar. Chem. 63, 119–129.
Murray J. W., Codispoti L. A., and Frienderich G. E. (1995) Oxidation-reduction environments: The suboxic zone of the Black Sea. In Aquatic Chemistry: Interfacial and Interspecies Processes (ed. C. P. Huang et al.), 244, pp. 157–176. American Chemical Society.
Nelson Y., Lion L. W., Shuler M. and Ghiorse W. (2002) Effect of oxide formation mechanisms on lead adsorption by biogenic manganese (hydr)oxides, iron (hydr)oxides, and their mixtures. Environ. Sci. Technol. 36, 421–425.
Perez-Benito J. F., Brillas E. and Pouplana R. (1989) Identification of a soluble form of colloidal manganese (IV). Inorg. Chem. 28, 390–392.
Perez-Benito J. F, Arias C. and Amat E. (1996) A kinetic study of the reduction of colloidal manganese dioxide by oxalic acid. J. Colloid Interface Sci. 177, 288–297.
Rozan T. F. and Luther III G. W. (2002) An anion chromatography/ultraviolet detection method to determine nitrite, nitrate, and sulfide concentrations in saline (pore)waters. Mar. Chem. 77, 1–6.
Schulz H. D., Dahmke A., Schinzel T., Wallmann K. and Zabel M. (1994) Early diagentic processes, fluxes, and reaction rates in sediments of the South Atlantic. Geochim. Cosmochim. Acta. 58, 2041–2060.
Shriver D. F., Atkins R., and Langford C. H. (1994) Inorganic Chemistry. 2nd ed. W. H. Freeman and Company, New York.
Skoog D. A., West D. M., and Holler F. J. (1992) Fundamentals of Analytical Chemistry. Saunders College Publishing, Fort Worth, TX.
Stone A. T. (1987) Reductive dissolution of manganese (III/IV) oxides by substituted phenols. Environ. Sci. Technol. 21, 979–988.
Stone A. T. and Morgan J. J. (1984a) Reduction and dissolution of manganese (III) and manganese(IV) oxides by organics: 1. Reaction with hydroquinone. Environ. Sci. Technol. 18, 450–456.
Stone A. T. and Morgan J. J. (1984b) Reduction and dissolution of manganese (III) and manganese(IV) oxides by organics: 2. Survey of the reactivity of organics. Environ. Sci. Technol. 18, 617–624.
Sorensen J., Jorgensen K. S., Colley S., Hydes D. J., Thomson, J. and Wilson T. R. S. (1987) Depth localization of denitrification in a deep-sea sediment from the Madeira Abyssal Plain. Limnol. Oceanogr. 32, 758–762.
Vandenabeele J., Vande Woestyne M., Houwen F., Germonpre R., Vandesande D. and Berstraete W. (1995) Role of autotrophic nitrifiers in biological manganese removal from ground water containing manganese and ammonium. Microbial Ecol. 29, 83–98.
Wilczak A, Knocke W. R., Hubel R. E. and Aieta E. M. (1993) Manganese control during ozonation of water containing organic compounds. J. Amer. Water Works Association 85, 98–104.
Xyla A. G., Sulzberger B., Luther G. W., Hering J. G., Van Cappellen P. and Stumm W. (1992) Reductive dissolution of manganese(III,IV) (hydr)oxides by oxalate: The effect of pH and light. Langmuir 8, 95–103.
Yao W. and Millero F. J. (1993) The rate of sulfide oxidation by δMnO2 in seawater. Geochim. Cosmochim. Acta 57, 3359–3365.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Luther, G.W., Popp, J.I. Kinetics of the Abiotic Reduction of Polymeric Manganese Dioxide by Nitrite: An Anaerobic Nitrification Reaction. Aquatic Geochemistry 8, 15–36 (2002). https://doi.org/10.1023/A:1020325604920
Issue Date:
DOI: https://doi.org/10.1023/A:1020325604920