Abstract
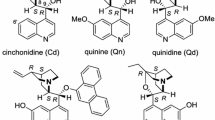
Cinchonidine and cinchonine have been grafted onto pure silica MCM-41. It has been shown that both supported alkaloids are active catalysts for the Michael addition of ethyl 2-oxocyclopentanecarboxylate and methyl vinyl ketone, anchored cinchonidine being more active and enantioselective than anchored cinchonine. The study of the influence of the polarity of the solvent and reaction temperature on the optical induction shows that there is not a direct correlation between solvent polarity and enantioselectivity, and the maximum optical yield was obtained between 278 and 273 K.
Similar content being viewed by others
References
S.C. Stinson, Chem. Engng News 28 (1992) 46.
G.M. Ramos Tombo and D. Bellus, Angew. Chem. 103 (1991) 1219.
R. Noyori, ChemTech 22 (1992) 366.
J.F. Nicoud and R.J. Twieg, in: Nonlinear Optical Properties of Organic Molecules and Crystals, eds. D.S. Chemla and J. Zyss (Academic Press, Orlando, 1987) p. 227.
H.U. Blaser, Chem. Rev. 92 (1992) 935.
R.A. Sheldon, Chirotechnology (Marcel Dekker, New York, 1993).
A. Tungler, T. Mathe, J. Petro and T. Tarnai, J. Mol. Catal. 61 (1990) 259; A. Tungler, T. Mathe, J. Petro and T. Tarnai, J. Mol. Catal. 67 (1991) 277; H.U. Blaser, Tetrahedron Asym. 2 (1991) 843; B. Minder, T. Mallat, P. Skrabal and A. Baiker, Catal. Lett. 29 (1994) 115; B. Minder, T.M. Shürch, T. Mallat and A. Baiker, Catal. Lett. 31 (1995) 143.
S. Itsuno and J.M.J. Frechet, J. Org. Chem. 52 (1987) 4140; K. Soai, S. Niwa and M. Watanabe, J. Org. Chem. 53 (1988) 927.
M.J. Sabater, A. Corma, A. Doménech, V. Fornés and H. García, Chem. Commun. (1997) 1285; S.B. Ogunwuni and T. Bein, Chem. Commun. (1997) 901.
A. Corma, M. Iglesias, C. del Pino and F. Sánchez, J. Chem. Soc., Chem. Commun. (1991) 1253.
A. Corma, M. Iglesias, C. del Pino and F. Sánchez, J. Organomet. Chem. 431 (1992) 233.
A. Corma, M. Iglesias, J.P. Obisco and F. Sánchez, in: Chiral Reactions in Heterogeneous Catalysis, eds. G. Jannes and V. Dubois (Plenum Press, New York and London, 1995) p. 179.
U. Nagel and E. Kingel, J. Chem. Soc., Chem. Commun. (1986) 1098.
K. Sosai, M. Watanabe and A. Yamamoto, J. Org. Chem. 55 (1990) 4832.
D. Brunel and P. Sutra, in: 3rd International Symposium on Supported Reagents and Catalysts in Chemistry (Royal Society of Chemistry, Limerick, Ireland, 1998) p. 54.
M. Lasperas, N. Bellocq, D. Brunel and P. Moreau, Tetrahedron Asym. 9 (1998) 3053.
N. Bellocq, D. Brunel, M. Lasperas and P. Moreau, Stud. Surf. Sci. Catal. 108 (1997) 485.
S. Abramson, M. Lasperas, A. Galarneau, D. Desplantier-Giscard and D. Brunel, Chem. Commun. (2000) 1773.
B. Langstrom and G. Bergson, Acta Chem. Scand. 27 (1973) 3118.
B.E. Rossiter and N.M. Swingle, Chem. Rev. 92 (1992) 771.
Y. Tamai, A. Kamifuku, E. Koshiishi and S. Miyano, Chem. Lett. (1995) 957, and references cited therein; R. Noyori, in: Asymmetric Catalysis in Organic Synthesis (Wiley, New York, 1994) p. 241; M. Wills and H. Tye, J. Chem. Soc., Perkin Trans. I (1999) 1109.
H. Sasai, T. Arai, Y. Satow, K.N. Houk and M. Shibasaki, J. Am. Chem. Soc. 117 (1995) 6194.
P. Bakó, L. Töke, A. Szöllösy and P. Bombicz, Heteroatom. Chem. 8 (1997) 333.
P. Bakó, Z. Bajor and L. Töke, J. Chem. Soc., Perkin Trans. 1 (1999) 3651.
P. Bakó, T. Kiss and L. Töke, Tetrahedron Lett. 38 (1997) 7259.
D.J. Cram and G.D.Y. Sogah, J. Chem. Soc., Chem. Commun. 13 (1981) 625.
H. Wynberg and R. Helder, Tetrahedron Lett. (1975) 4057; (b) K. Hermann and H. Wynberg, J. Org. Chem. 44 (1979) 2738; (c) H. Brunner and B. Hammer, Angew. Chem. Int. Ed. Engl. 23 (1984) 312; (d) H. Brunner and C. Krumey, J. Mol. Catal. 142 (1999) 7; (e) A. Latvala, S. Stanchev, A. Linden and M. Hesse, Tetrahedron Asym. 4 (1993) 173.
M. Iglesias-Hernández and F. Sánchez-Alonso, Stud. Surf. Sci. Catal. 130 (2000) 3393.
C. Krumey, PhD Thesis, University of Regensburg, 1995.
H. Buschmann, H.D. Scharf, N. Hoffmann and P. Esser, Angew. Chem. Int. Ed. Engl. 30 (1991) 477; (b) D. Heller, H. Buschmann and H.D. Scharf, Angew. Chem. Int. Ed. Engl. 35 (1996) 1852; (c) H. Zhang and K.S. Chan, J. Chem. Soc., Perkin Trans. I (1999) 381; (d) V.V. Krotov, S.M. Staroverov, P.N. Nesterenko and G.V. Lisichkin, Zh. Obshch. Khim. 57 (1987) 1187. (e) B. Giese, Angew. Chem. Ed. Int. Eng. 16 (1977) 125; (f) H.D. Scharf, J. Am. Chem. Soc., 111 (1989) 5367; (g) K. J. Hale and J.H. Ridd, J. Chem. Soc., Chem. Commun. (1995) 357.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Corma, A., Iborra, S., Rodríguez, I. et al. MCM-41 Heterogenized Chiral Amines as Base Catalysts for Enantioselective Michael Reaction. Catalysis Letters 82, 237–242 (2002). https://doi.org/10.1023/A:1020531315091
Issue Date:
DOI: https://doi.org/10.1023/A:1020531315091