Abstract
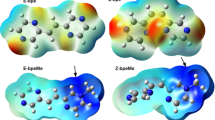
The photophysical properties of three newly synthesized pyrazoloquinolines, composed of N,N-dimethylaniline as donor subunit and various substituted forms of the acceptor pyrazoloquinoline (DPPQ), were investigated by absorption as well as by stationary and time resolved fluorescence spectroscopy. These compounds show generally highly efficient emission in nonpolar and medium polar solvents; the dipole moment of the emitting state increases and the quantum yield decreases with solvent polarity. These results are explained by state reversion in polar solvents: At low polarities emission originates from a state localized on the DPPQ moiety, whereas in the high-polarity regime the next excited state of charge transfer character, in which an electron is promoted from the amino nitrogen lone pair into an excited orbital of the DPPQ moiety, becomes the fluorescent state. This view is corroborated by semiempirical calculations including the solvent reaction field, low-temperature fluorescence measurements, and the observation of effects of protonation on the spectroscopic and photophysical properties.
Similar content being viewed by others
REFERENCES
W. Rettig (1986) Angew. Chem. Int. Ed. Engl. 25, 971.
W. Rettig (1994) in J. Mattay (Ed.), Topics in Current Chemistry, Springer, Berlin, p. 253.
Z. R. Grabowski, K. Rotkiewicz, A. Siemiarczuk, D. J. Cowley, and W. Baumann (1979) Nouv. J. Chim. 3, 443.
S. R. Marder, B. Kippelen, A. K-Y. Jen, and N. Peyghambarian (1997) Nature 388, 845.
J. Herbich and A. Kapturkiewicz (1991) Chem. Phys. 158, 143.
J. Herbich and A. Kapturkiewicz (1993) Chem. Phys. 170, 221.
A. Kapturkiewicz, J. Herbich, J. Karpiuk, and J. Nowacki (1997) J. Phys. Chem. A 101, 2332.
G. Grabner, K. Rechthaler, and G. Köhler (1998) J. Phys. Chem. A 102, 689.
K. Rotkiewicz, K. Rechthaler, A. Puchała, D. Rasała, S. Styrcz, and G. Köhler (1996) J. Photochem. Photobiol. A Chem. 98, 15.
A. B. J. Parusel, R. Schamschule, D. Piorun, K. Rechthaler, A. Puchała, D. Rasała, K. Rotkiewicz, and G. Köhler (1997) J. Mol. Struct. (THEOCHEM) 419, 63.
A. B. J. Parusel, R. Schamschule, and G. Köhler (1997) Ber. Bunsenges. Phys. Chem. 101, 1836.
K. Rechthaler, K. Rotkiewicz, A. Danel, P. Tomasik, and K. Khatchatryan (1997) J. Fluoresc. 7, 301.
D. Tomasik, P. Tomasik, and R. A. Abramovitch (1983) J. Heterocycl. Chem. 20, 1539.
A. Danel, K. Khatchatryan, and P. Tomasik (unpublished).
J. Jasny (1978) J. Luminescence 17, 149.
M. J. S. Dewar, E. G. Zoebisch, E. F. Healy, and J. J. P. Stewart (1985) J. Am. Chem. Soc. 107, 3902.
G. Rauhut, A. Alex, J. Chandrasekhar, T. Steinke, W. Sauer, B. Beck, M. Hutter, P. Gedeck, and T. Clark (1997) VAMP6.1, Oxford Molecular, Oxford.
G. Rauhut, T. Clark, and T. Steinke (1993) J. Am. Chem. Soc. 115, 9174.
G. Köhler, P. Wolschann, and K. Rotkiewicz (1992) Proc. Indian Acad. Sci. (Chem. Sci.) 104, 197.
T. Clark and J. Chandrasekhar (1993) Israel J. Chem. 33, 435.
D. R. Lide (ed.), CRC Handbook of Chemistry and Physics (1992/93), 73rd ed., CRC Press, Boca Raton, FL, pp. 6–167; M. Maroncelli (1993) J. Mol. Liq. 57, 1.
W. W. Simons (Ed.) (1979) The Sadtler Handbook of Ultraviolet Spectra, Sadtler Research Laboratories, Philadelphia, p. 154.
Rights and permissions
About this article
Cite this article
Parusel, A.B.J., Rechthaler, K., Piorun, D. et al. Fluorescence Properties of Donor–Acceptor-Substituted Pyrazoloquinolines. Journal of Fluorescence 8, 375–387 (1998). https://doi.org/10.1023/A:1020580700692
Issue Date:
DOI: https://doi.org/10.1023/A:1020580700692