Abstract
Purpose. Biodistribution of drugs in the eye is central to the efficacy of pharmaceutical ocular therapies. Of particular interest to us is the effect of intravitreal transport on distribution of controlled-released drugs within the vitreous.
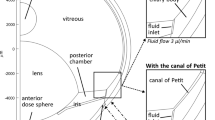
Methods. A computer model was developed to describe the three-dimensional convective-diffusive transport of drug released from an intravitreal controlled release source. Unlike previous studies, this work includes flow of aqueous from the anterior to the posterior of the vitreous. The release profile was based on in vitro release of gentamicin from poly(L-lactic acid) microspheres into vitreous.
Results. For small drugs, convection plays a small role, but for large (slower diffusing) drugs, convection becomes more important. For the cases studied, the predicted ratio of drug reaching the retina to drug cleared by the aqueous humor was 2.4 for a small molecule but 13 for a large molecule. Transport in neonatal mouse eye, in contrast, was dominated by diffusion, and the ratio decreased to 0.39.
Conclusions. The interaction among convection, diffusion, and geometry causes significant differences in biodistribution between large and small molecules or across species. These differences should be considered in the design of delivery strategies or animal studies.
Similar content being viewed by others
REFERENCES
W. M. Hart. Adler's Physiology of the Eye, Mosby-Year Book, Chicago, Illinois, 1992.
W. H. Stern, G. P. Lewis, P. A. Erickson, C. J. Guerin, D. H. Anderson, S. K. Fisher, and J. J. O'Donnell. Fluorouracil Therapy for Proliferative Vitreoretinopathy After Vitrectomy. Am. J. Ophthalmol. 96:33-42 (1983).
G. M. Bleeker. N. J. V. Haeringen, E. R. Mass, and E. Glasius. Selective Properties of the Vitreous Barrier. Exp. Eye Res. 7:37-46 (1968).
F. Q. Liang and R. S. Viola. M. d. Cerro, and V. Aquavella. Noncross-linked Collagen Discs and Cross-linked Collagen Shields in the Delivery of Gentamicin to Rabbits Eyes. Invest. Ophthalmol. Vis. Sci. 33:2194-2198 (1992).
G. G. Giordano, M. F. Refojo, and M. H. Arroyo. Sustained Delivery of Retinoic Acid from Microspheres of Biodegradable Polymer in PVR. Invest. Ophthalmol. Vis. Sci. 34:2743-2751 (1993).
H. Miyamoto, Y. Ogura, M. Hashizoe, N. Kunou, Y. Honda, and Y. Ikada. Biodegradable Scleral Implant for Intravitreal Controlled Release of Fluconazole. Curr. Eye Res. 16:930-935 (1997).
S. C. Pflugfelder, E. Hernandez, and S. J. Fliesler. and J. Alvarez. Intravitreal Vancomycin. Retinal Toxicity, Clearance, and Interaction with Gentamicin. Arch. Ophthalmol. 105:831-837 (1987).
R. T. Falk, T. W. Randolph, J. Meyer, R. Kelly, and M. Manning. Controlled Release of Ionic Compounds from Poly (L-lactide) Microspheres Produced by Precipitaion with a Compressed Antisolvent. J. Control. Release 44:77-85 (1997).
J. D. Meyer, R. F. Falk, R. M. Kelley, J. E. Shively, S. J. Withrow, W. S. Dernell, D. J. Kroll, T. W. Randolph, and M. C. Manning. Preparation and In Vitro Characterization of Gentamicin-Impregnated Biodegradable Beads Suitable for Treatment of Osteomyelitis. J. Pharm. Sci. 87:1149-1154 (1997).
W. Dernell, S. Withrow, M. Manning, C. Kuntz, R. Dewell, F. Garry, B. Powers, J. Shively, R. Falk, and T. Randolph. In Vivo Evaluation of Gentamicin-Impregnated Polylactic Acid Beads Implanted in Sheep. J. Bioact. and Compat. Polym. 16:119-135 (2001).
W. Dernell, C. Gentry-Weeks, M. Manning, B. Powers, R. Park, M. Lafferty, C. Kuntz, J. Shively, R. Falk, J. Meyer, T. Randolph, and S. Withrow. In Vivo Evaluation of Anibiotic Impregnated beads in a Rat Osteomyelitis Model. J. Bioact. Compat. Polym. 16:235-250 (2001).
J. Xu. Controlled release and the concentration distribution of the drug in the vitreous humor. M. S. Thesis in Chemical Engineering, University of Colorado, Boulder, Colorado, 1999.
J. Xu, J. J. Heys, T. W. Randolph, and V. H. Barocas. Permeability, and diffusion in the vitreous humor: Implications for controlled drug delivery. Pharm. Res. 17:664-669 (2000).
A. Ohtori and K. J. Toko. In Vivo/In Vitro Correlation of Intravitreal Delivery of Drugs With the Help of Computer Simulation. Biol. Pharm. Bull. 17:283-290 (1994).
M. Araie and D. Maurice. The Loss of Flourescein, Flourescein Glucuronide and Flourescein Isothiocyanate Dextran from the Vitreous by the Anterior and Retinal Pathways. Exp. Eye Res. 52:27-39 (1991).
S. Friedrich, Y. Cheng, and B. Saville. Drug Distribution in the Vitreous Humor of the Human Eye: The Effect of Intravitreal Injection Position and Volume. Curr. Eye Res. 16:663-669 (1997).
I. Fatt and B. Hedbys. Flow of water in the sclera. Exp. Eye Res. 10:243-249 (1970).
W. G. Robison, T. Kuwabara, and J. Zwaan. Eye research. In H. L. Foster, J. D. Small, and J. G. Fox (eds.), The Mouse in Biomedical Research. Volume IV. Experimental Biology and Oncology, Academic Press, New York, 1982 pp. 69-89.
P. A. Pearson, G. J. Jaffe, D. F. Martin, G. J. Cordahi, H. Grossniklaus, E. T. Schmeisser, and P. Ashton. Evaluation of a delivery system providing long-term release of cyclosporine. Arch. Ophthalmol. 114:311-317 (1996).
C. B. Engler, B. Sander, M. Larsen, P. Dalgaard, and H. Lund-Andersen. Fluorescein Transport Across the Human Blood Retina Barrier in the Direction Vitreous to Blood-Quantitative Assessment in-Vivo. Acta Ophthalmol. (Copenh.) 72:655-662 (1994).
B. Moldow, B. Sander, M. Larsen, and H. Lund-Andersen. Effects of acetazolamide on passive and active transport of fluorescein across normal BRB. Invest. Ophthalmol. Vis. Sci. 40:1771-1775 (1999).
D. Maurice. Flow of water between aqueous and vitreous compartments in the rabbit eye. Am. J. Physiol. 252:F104-F108 (1987).
S. Tsuboi and J. E. Pederson. Effect of plasma osmolality and intraocular pressure on fluid movement across the blood-retinal barrier. Invest. Ophthalmol. Vis. Sci. 29:1747-1749 (1988).
M. Johnson and K. Erickson. Mechanisms and routes of aqueous humor drainage. In D. M. Albert and F. A. Jakobiec (eds.), Glaucoma, W.B. Saunders: Philadelphia, Pennsylvania, 2000 pp. 2577-2594.
Y. Sugiura and M. Araie. Effects of intraocular pressure change on movement of FITC-dextran across vitreous-aqueous interface. Jpn. J. Ophthalmol. 33:441-450 (1989).
H. M. Cheng, K. K. Kwong, J. Xiong, and B. T. Woods. Visualization of water movement in the living rabbit eye. Graefes Arch. Clin. Exp. Ophthalmol. 230:62-65 (1992).
S. Koyano, M. Araie, and S. Eguchi. Movement of fluorescein and its glucuronide across retinal pigment epithelium-choroid. Invest. Ophthalmol. Vis. Sci. 34:531-538 (1993).
A. Yoshida, M. Kojima, and S. Ishiko. Inward and outward permeability of the blood-retinal barrier. In J. Cunha-Vaz and E. Leite (eds.). Ocular Fluorophotometry and the Future, Kugler & Ghedini Publishers, Amsterdam, 1989, pp. 89-97.
A. Yoshida, S. Ishiko, and M. Kojima. Outward Permeability of Blood-Retinal Barrier. Graefes Arch. Clin. Exp. Ophthalmol. 230:78-83 (1992).
S. Friedrich, B. Saville, and Y. Cheng. Finite Element Modeling of Drug Distribution in the Vitreous Humor of the Rabbit Eye. Annals Biomed. Eng. 25:303-314 (1997).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stay, M.S., Xu, J., Randolph, T.W. et al. Computer Simulation of Convective and Diffusive Transport of Controlled-Release Drugs in the Vitreous Humor. Pharm Res 20, 96–102 (2003). https://doi.org/10.1023/A:1022207026982
Issue Date:
DOI: https://doi.org/10.1023/A:1022207026982