Abstract
Purpose. The aim of this work was to develop and characterize a highly loaded nanoparticulate system based on amphiphilic β-cyclodextrins (CDs) to facilitate the parenteral administration of poorly soluble antifungal model drugs bifonazole and clotrimazole.
Methods. Inclusion complexes were characterized with spectroscopic techniques. Particle size distribution of nanospheres were determined by photon correlation spectroscopy (PCS). Nanospheres were assessed for hemolytic activity. Entrapped and released drug quantities were determined and minimum inhibitory concentration (MIC) values of drugs, amphiphilic β-CDs, and drug loaded nanospheres were evaluated.
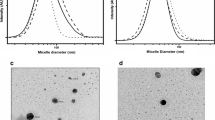
Results. 1:1 inclusion complexes of model drugs with amphiphilic β-CDs gave nanospheres <300 nm (polydispersity index < 0.15) by nanoprecipitation technique without using surfactants. By direct preparation from preformed inclusion complexes, loading was increased 2- to 8-fold depending on CD type and loading technique. Conventionally loaded CD nanospheres displayed immediate release whereas preloaded and highly loaded nanospheres liberated model drugs over a period of 1 h reducing the initial burst effect. MIC values of bifonazole and clotrimazole were lowered significantly when associated to amphiphilic β-CD nanospheres.
Conclusion. Amphiphilic β-CDs form nonsurfactant, highly loaded nanospheres with lower hemolytic activity than that of natural CDs directly from inclusion complexes. They enhanced solubility and subsequently therapeutic efficacy of the model drugs.
Similar content being viewed by others
REFERENCES
D. Duchene, D. Wouessidjewe, and G. Ponchel. Cyclodextrins and carrier systems. J. Control. Release 62:263-268 (1999).
D. Woussidjewe, M. Skiba, F. Leroy-Lechat, E. Lemos-Senna, F. Puisieux, and D. Duchene. A new concept in drug delivery based on skirt-shaped cyclodextrin aggregates, present state and future prospects. STP Pharma Sci. 6:21-28 (1996).
P. C. Tchoreloff, M. M. Boissonnade, A. W. Coleman, and A. Baszkin. Amphiphilic monolayers of insoluble cyclodextrins at the water/air interface. Surface pressure and surface potential studies. Langmuir 11:191-196 (1995).
C. Ringard-Lefebvre, A. Bochot, E. Memişoğlu, D. Charon, D. Duchene, and A. Baszkin. Interfacial behavior of the oil/water system in the presence of spread amphiphilic cyclodextrins. Coll. Surf. B. Biointerfaces 25:109-117 (2002).
E. Memişoğlu, A. Bochot, D. Charon, A. A. Hıncal, and D. Duchene. Characterization of nanocapsules composed of amphiphilic beta-cyclodextrins modified on the primary face. Eur. J. Pharm. Sci. 11(Suppl):S29(2001).
E. Memişoğlu, A. Bochot, M. Şen, D. Charon, D. Duchene, and A. A. Hıncal. Amphiphilic β-cyclodextrins modified on the primary face: Synthesis, characterization and evaluation of their potential as novel excipients in the preparation of nanocapsules. J. Pharm. Sci. 91:1214-1224 (2002).
D. O. Thompson. Cyclodextrins-enabling excipients. Their present and future use in pharmaceutics (review). CRC Crit. Rev. Ther. Drug Carrier Syst. 14:1-104 (1997).
H. Van Doorne, E. H. Bosch, and C. F. Lerk. Formation of antimicrobial activity of complexes of β-cyclodextrin and antimycotic imidazole derivatives. Pharm. Weekblad (Sci.) 10:80-85 (1988)
A. R. Hedges. Industrial applications of cyclodextrins. Chem. Rev. 98:2035-2044 (1998).
H. Fessi, J.P. Devissaguet, C. Thies. Process for the preparation of dispersible colloidal systems of a substance in the form of nanospheres. US Patent, 5,118,528 (1988)
National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 4th Ed, Approved Standard. M7 A4, 17(2), NCCLS, Villanova, PA (1997).
J. L. Ford and P. Timmins. Pharmaceutical Thermal Analysis, Ellis Horwood Limited. Chichester, 1989
H. Takahashi, Y. Irinatsu, S. Kozuka, and W. Tagaki. Host-guest complexation of a lipophilic heptakis(6-dodecylamino-6-deoxy)-β-cyclodextrin with nitrophenols in chloroform. Mem. Fac. Eng. Osaka City Univ. 26:93-99 (1985).
H. Nakahara, H. Tanaka, K. Fukuda, M. Matsumoto, and W. Tagaki. Selective inclusion of naphthalene derivatives by monolayers of amphiphilic cyclodextrins at the air-water interface. Thin Solid Films 284-285:687-690 (1996).
N. Morin, G. Crini, C. Cosentino, J. Milet, and J. Vebrel. JC, Rouland. Formation of two particular structures between β-cyclodextrin and bifonazole: β-cyclodextrin-bifonazole and (β-cyclodextrin)i _bifonazole (where 2<i<3). J. Chem. Soc. Perkin-Trans. 2:2647-2651 (1999).
E. Lemos-Senna, D. Wouesidjewe, S. Lesieur, F. Puisieux, G. Couarrazze, and D. Duchene. Evaluation of the hydrophobic drug loading characteristics in nanoprecipitated amphiphilic cyclodextrin nanospheres. Pharm. Dev. Technol. 3:1-10 (1998).
E. Lemos-Senna, D. Wouessidjewe, S. Lesieur, and D. Duchene. Preparation of amphiphilic cyclodextrin nanospheres using the emulsion solvent evaporation method, influence of the surfactant on preparation and hydrophobic drug loading. Int. J. Pharm. 170:119-128 (1998).
F. Hirayama, M. Yamanaka, T. Horikawa, and K. Uekama. Characterization of peracetylated β-cyclodextrins with different chain lengths as a novel sustained release carrier for water-soluble drugs. Chem. Pharm. Bull. 43:130-136 (1995).
F. Hirayama and K. Uekama. Cyclodextrin-based controlled drug release system. Adv. Drug Del. Rev. 36:125-144 (1995).
J. Szejtli, T. Cserhati, and M. Szögyi. Interactions between cyclodextrins and cell membrane phospholipids. Carbohyd. Polym. 6:35-49 (1986).
H. Yamaguchi, T. Hiratani, and M. Plempel. In vitro studies of a new imidazole antimycotic, bifonazole in comparison with clotrimazole and miconazole. Arzneim. Forsch./Drug Res. 33:546-551 (1983)
M. Pedersen, S. Bjerregaard, J. Jacobsen, and A. Mehlsen Sorensen. A genuine clotrimazole-Γ-cyclodextrin inclusion complex. Isolation, antimycotic activity, toxicity and unusual dissolution rate. Int. J. Pharm. 176:121-131 (1998).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Memişoğlu, E., Bochot, A., Özalp, M. et al. Direct Formation of Nanospheres from Amphiphilic β-Cyclodextrin Inclusion Complexes. Pharm Res 20, 117–125 (2003). https://doi.org/10.1023/A:1022263111961
Issue Date:
DOI: https://doi.org/10.1023/A:1022263111961