Abstract
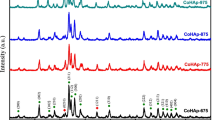
Powders of hydroxyapatite (HA), partially fluoride-substituted hydroxyapatite (fHA), and fluorapatite (FA) were synthesized in house using optimum methods to achieve relatively pure powders. These powders were assessed by the commonly used bulk techniques of X-ray diffraction (XRD), Fourier transform infra-red (FTIR) and FT-Raman spectroscopies, inductively coupled plasma atomic emission spectroscopy (ICP-AES), and F-selective electrode. In addition, the current study has employed transmission electron microscopy (TEM), involving morphological observation, electron diffraction and energy-dispersive X-ray spectrometry (EDX), as an effective analytical technique to evaluate the powders at a microscopic level. The HA and fHA particles were elongated platelets about 20×60 nm in size, while FA particles were over twice this size. Calcination of the HA and fHA powders at 1000 °C for 1 h resulted in increased grain size and crystallinity. The calcined fHA material appeared to possess a crystal structure intermediate between HA and FA, as evidenced by the (3 0 0) peak shift in XRD, as well as by the position of the hydroxyl bands in the FTIR spectra. This result was consistent with electron diffraction of individual particles. Small levels of impurities in some of the powders were identified by EDX and electron diffraction, and the carbonate content was detected by FTIR. The use of TEM in conjunction with the bulk techniques has allowed a more thorough assessment of the apatites, and has enabled the constituents in these closely related apatite powders to be identified.
Similar content being viewed by others
References
R. Z. Legeros and J. P. Legeros, in An Introduction to Bioceramics, edited by L. L. Hench and J. Wilson (World Scientific, Singapore, 1993).
M. Okazaki, Y. Miake, H. Tohda, T. Yanagisawa, T. Matsumoto and J. Takahashi, Biomater. 20 (1999) 1421.
J. C. Elliott, in Studies in Inorganic Chemistry, Vol. 18 (Elsevier, Amsterdam, 1994).
G. Penel, G. Leory, C. Rey, B. Sombret, J. P. Huvenne and E. Bres, J. Mater. Sci. Mater. Med. 8 (1997) 271.
C. B. Baddiel and E. E. Berry, Spectrochemica Acta 22 (1966) 1407.
Y. Liu, P. Comodi and P. Sassi, N. Jb. Miner. Abh. 174 (1998) 211.
F. Freund and R. M. Knobel, J. Chem. Soc., Dalton Trans. (1977) 1136.
A. Baumer, M. Ganteaume and W. E. Klee, Bull. Mineral. 108 (1985) 145.
M. Braun, P. Hartmann and C. Jana, J. Mater. Sci. Mater. Med. 6 (1995) 150.
L. J. Jha, S. M. Best, J. C. Knowles, I. Rehman, J. D. Santos and W. Bonfield, ibid. 8 (1997) 185.
M. Wei, A. J. Ruys, B. K. Milthorpe and C. C. Sorrell, J. Biomed. Mater. Res. 45 (1999) 11.
H.-J. Kleebe, E. F. Bres, D. Bernache-Assolant and G. Ziegler, J. Am. Ceram. Soc. 80 (1997) 37.
H. Ji and P. M. Marquis, J. Mater. Sci. Letters 10 (1991) 132.
E. I. Suvorova, F. Christensson, H. E. Lundager Madsen and A. A. Chernov, J. Cryst. Growth 186 (1998) 262.
E. I. Suvorova and P. A. Buffat, J. Microsc. 196 (1999) 46.
M. Jarcho, C. H. Bolen, M. B. Thomas, J. Boick, J. F. Kay and R. H. Doremus, J. Mater. Sci. 11 (1976) 2027.
M. Hirano, H. Takeuchi and M. Ono, in Sintering'87 Volume 2, Proc. of the International Institute for the Science of Sintering Symposium, edited by S. Somiya, M. Shimada, M. Yoshimura and R. Watanabe (Elsevier, Amsterdam, Holland, 1987).
T. Futagami and T. Okamoto, J. Ceram. Soc. Jap. 95 (1987) 775.
E. D. Eanes and A. W. Hailer, Calcif. Tissue Int. 63 (1998) 250.
M. Okazaki, H. Tohda, T. Yanagisawa, M. Taira and J. Takahashi, Biomater. 19 (1998) 919.
A. Traveria-Cros, M. Cuevas-Diarte, F. Plana-Lievat and M. Font-Altaba, Acta Geol. Hisp. 15 (1980) 15.
R. Fabian, I. Kotsis, P. Zimany and P. Halmos, Talanta 46 (1998) 1273.
E. J. Duff and J. L. Stuart, Anal. Chem. Acta. 52 (1970) 155.
J. L. Labar, in Proc. of EUREM 12, Volume III, edited by L. Frank and F. Ciampor (Brno, Hungary, 2000).
J. D. B. Featherstone, S. Pearson and R. Z. Legeros, Caries Res. 18 (1984) 63.
L. Grondahl, L. Rintoul, M. Wei, E. Wentrup-Byrne and J. H. Evans. Work in progress.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wei, M., Evans, J.H., Bostrom, T. et al. Synthesis and characterization of hydroxyapatite, fluoride-substituted hydroxyapatite and fluorapatite. Journal of Materials Science: Materials in Medicine 14, 311–320 (2003). https://doi.org/10.1023/A:1022975730730
Issue Date:
DOI: https://doi.org/10.1023/A:1022975730730