Abstract
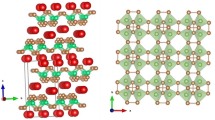
K2.6Nb11.6O30, Mr = 1659.33 g ⋅ mol−1, crystallizes in the tetragonal system, space group P4/mbm, Z = 1. The lattice parameters are a = 12.549(3) Å, c = 3.978(2) Å, V = 626.4(4) Å3, D x _{x} = 4.398 g ⋅ cm−3. The crystal structure was solved and refined from 413 independent reflections, obtained by X-ray diffraction on a single crystal. The final R index and weighted R w index are 0.0500 and 0.1178, respectively. The three-dimensional network of K2.6Nb11.6O30 is similar to that of tetragonal tungsten bronze with K and Nb partially occupying the interstitial pentagonal and trigonal sites. The most interest of this structure is the planar pentagonal coordination of Nb atoms.
Similar content being viewed by others
References
Magneli, A. Ark. Kemi 1949, 1, 213.
Abrahams, S.C.; Jamieson, P.B.; Bernstein, J.L. J. Chem. Phys. 1971, 54, 2355.
Bonner, W.A.; Grodkiewiez, W.H.; Van Uitert, L.G. J. Cryst. Growth 1967, 1, 318.
Van Uitert, L.G.; Levinstein, H.J.; Rubin, J.J.; Capio, C.D.; Dearborn, E.F.; Bonner, W.A. Mater. Res. Bull. 1968, 3, 47.
Jamiesson, P.B.; Abrahams, S.C.; Bernstein, J.L. J. Chem. Phys. 1968, 48, 5048.
Farrugia, L.J. J. Appl. Crystallogr. 1999, 32, 837–838.
Sheldrick, G.M. SHELXS86, Program for Crystal Structure Solution; University of Göttingen: Göttingen, Germany, 1985.
Sheldrick, G.M. SHELXL93, Program for Crystal Structure Refinement; University of Göttingen: Göttingen, Germany, 1993.
Banks, E.; Wold, A. In Preparative Inorganic Reactions; Jolly, W.L., Ed.; Interscience: New York, 1968; Vol. 4, p. 237.
Hagenmuller, P. Progress in Solid State Chemistry; Pergamon: New York, 1971; Vol. 5, p. 71.
Sienko, M.J. In Non-Stoichometry Compounds; (Advances in Chemistry Series); Gould, R.F., Ed.; American Chemical Society: Washington, DC, 1963; p. 224.
Dickens, P.G.; Whittingham, M.S. Rev. Chem. Soc. 1968, 22, 30.
Hessen, B.; Sunshine, S.A.; Siegrist, T.; Fiory, A.T.; Waszczak, J.V. Chem. Mater. 1991, 3, 528–534.
D'yachenko, O.G.; Istomin, S. Ya.; Fedotov, M.M.; Antipov, E.V.; Svensson, G.; Nygren, M.; Holm, W. Mater. Res. Bull. 1997, 32(4), 409–419.
Chen, S.C.; Greenblatt, M. J. Solid State Chem. 1993, 104, 353–358.
Kumada, N.; Kinomura, N. Eur. J. Solid State Inorg. Chem. 1997, 34, 65–72.
Brik, F.; Enjalbert, R.; Roucau, C.; Galy, J. J. Solid State Chem. 1996, 122, 7–14.
Lenzo, P.V.; Spencer, E.G.; Ballman, A.A. Appl. Phys. Lett. 1967, 11, 23.
Jamieson, P.B.; Abrahams, S.C.; Bernstein, J.L. J. Chem. Phys. 1969, 50, 4352.
Geusic, J.E.; Levinstein, H.J.; Rubin, J.J.; Singh, S.; Van Uitert, L.G. Appl. Phys. Lett. 1967, 11, 269
Geusic, J.E.; Levinstein, H.J.; Rubin, J.J.; Singh, S.; Van Uitert, L.G. Appl. Phys. Lett. 1968, 12, 224.
Abrahams, S.C.; Jamieson, P.B.; Bernstein, J.L. J. Chem. Phys. 1971, 54, 2355–2364.
Jamieson, P.B.; Abrahams, S.C. Acta Crystallogr. Sect. B 1968, 24, 984.
Mironov, A.V.; Istomin, S. Ya.; D'yachenko, O.G.; Antipov, E.V. J. Solid State Chem. 2001, 157, 1–7.
Brandt, R.; Mueller-Buschbaum, H. Monatshefte fuer Chemie und verwandte Teile anderer Wissenschaften 1986, 117, 1239–1244.
Lehmann, U.; Mueller-Buschbaum, H.Z. Anorg. Allg. Chem. 1981, 481, 7–12.
Chernaya, T.S.; Maksimov, B.A.; Verin, I.V.; Ivleva, L.I.; Simonov, V.I. Kristallografiya 1997, 42(3), 421–426.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ftini, M.M., Ayed, B. & Haddad, A. K2.6Nb11.6O30: a novel tetragonal bronze structure. Journal of Chemical Crystallography 33, 123–129 (2003). https://doi.org/10.1023/A:1023222825088
Issue Date:
DOI: https://doi.org/10.1023/A:1023222825088