Abstract
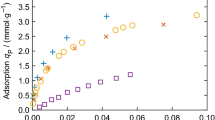
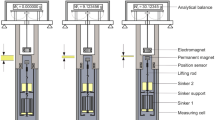
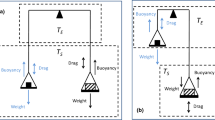
In sorption measurements, volumetric or gravimetric procedures are commonly used to determine the amount adsorbed. At low pressures, thermomolecular flow and pressure differences according to Knudsen's law disturb measurements. In volumetry, calibration of the dead space is required; in gravimetry, the influence of buoyancy has to be taken into account. In both cases, adsorption of the calibrating gas, usually helium, may disturb the measurements [1]. From the calibration measurements, the density of the sample can in principle be calculated. However, it has been observed in many experiments that its value depends on the calibrating gas.
Similar content being viewed by others
References
R. Staudt, G. Saller, M. Tomalla and J. U. Keller, Ber. Bunsenges. Phys. Chem., 97 (1993) 98.
E. Robens, G. Sandstede, G. Walter and G. Wurzbacher, 'Fluctuations of the weight indicated by a microbalance in the pressure range between 1 and 103 torr with the sample at a lower temperature than the beam'. In: C. H. Massen and H. J. van Beckum (Eds.): Vacuum Microbalance Techniques Vol. 7, Plenum, New York 70, p. 195.
C. H. Massen, E. Robens, J. A. Poulis and Th. Gast, Thermochim. Acta, 82 (1984) 43.
L. Ginoux and L. Bonnetain, Some problems about gas adsorption isotherm measurements by automated procedures in manometric devices. In: F. Rodriguez-Reinoso, J. Rouquerol, K. S. W. Sing and K. K. Unger (Eds.): Characterization of Porous Solids II. Elsevier, Amsterdam, 1991, p. 189.
E. L. Fuller, J. A. Poulis, A. W. Czanderna and E. Robens, Thermochim. Acta, 29 (1979) 315.
I. F. Homfray, Z. Phys. Chem., 74 (1910) 129.
J. M. Fernbacher and L. A. Wenzel, Ind. Eng. Chem. Fundam., 11 (1972) 457.
E. Robens, J. A. Poulis, C. H. Massen and K. K. Unger, Determination of the amount adsorbed from the gas phase in a porous solid. In: M. Ben Chanaa (Ed.): Proceedings of the XXVIth International Conference on Vacuum Microbalance Techniques, Marrakesh (Morocco), April 26–28, 1995, Université Cadi Ayyad, p. 21.
A. López-Peinado, J. Rivera-Utrilla and J. D. López-González, Adsorption Sci. & Technol., 2 (1985) 31.
M. Grebner, Synthese und Eigenschaften von Dodecasil 1H als vielseitige Wirtsmatrix Dissertation, Mainz, 1994.
H. Kuwabara, T. Suzuki and K. Kaneko, J. Chem. Soc. Faraday Trans., 87 (1991) 1915.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Robens, E., Keller, J.U., Massen, C.H. et al. Sources of Error in Sorption and Density Measurements. Journal of Thermal Analysis and Calorimetry 55, 383–387 (1999). https://doi.org/10.1023/A:1010195013633
Issue Date:
DOI: https://doi.org/10.1023/A:1010195013633