Abstract
Biseugenyl succinate (BEUS) and bis(4-maleimidephenyl) succinate (BMIS) were synthesized by the reaction of succinic acid (SA) with eugenol and the reaction of succinyl chloride with 4-hydroxyphenylmaleimide, respectively. A gelled mixture of BEUS and BMIS at a molar ratio of 1/1, 1/2 or 1/3 at 200 °C was compression-molded at 230 °C for 1 h to produce a cured BEUS/BMIS product. The properties of BEUS/BMIS were compared with the properties of 2,2′-diallyl bisphenol A (DABA)/4,4′-bismaleimidediphenylmethane (BMIM) cured at 230 °C for 1 h. The Fourier Transform Infrared (FTIR) analysis of the cured materials revealed that chain polymerization of allyl and maleimide groups occurred to produce BEUS/BMIS, whereas a stepwise ene reaction and subsequent chain polymerization in addition to an etherification reaction occurred to produce DABA/BMIM. The cured BEUS/BMIS (1/2, 1/3) showed a higher glass transition temperature and greater tensile strength than the corresponding DABA/BMIM.
Similar content being viewed by others
Introduction
In addition to bio-based thermoplastic polymers, such as polylactide, polyhydroxyalkanoate and cellulose acetate, thermosetting resins derived from renewable resources are increasing in prominence as sustainable and eco-efficient products that can replace the products based exclusively on petroleum feedstock.1, 2, 3, 4, 5, 6, 7, 8 It is important for the development of bio-based thermosetting resins that their essential characteristics such as superior thermal and chemical resistance, rigidity and durability as demonstrated by high crosslinking density be maintained and the drawbacks, such as brittleness and high curing temperature, be improved.5 Petroleum-based thermosetting bismaleimide resins are used as the matrix resins for multilayer printed circuit boards and advanced composite materials in the aerospace industry.9, 10, 11 For example, a commercial thermosetting bismaleimide resin, Matrimid 5292 (Ciba-Geigy, Basel, Switzerland), based on 2,2′-dially bisphenol A (DABA)/4,4′-diphenylmethanebismalemide (BMIM), is one of the leading matrix resins in carbon fiber composites for advanced aerospace application (Figure 1).12 Fourier Transform Infrared (FTIR) analysis of DABA has shown that a stepwise ene reaction and subsequent chain polymerization in addition to an etherification reaction occurs in the production of DABA/BMIM.13, 14 Although the fully cured DABA/BMIM possesses superior thermal stability and mechanical properties, its typical curing condition (230–250 °C/4–6 h) are too severe to allow it to be applied to a wide range of uses such as general purpose electronics and structural materials. We had already reported that the BMIM cured with tung oil or dehydrated castor oil at 200 °C for 2 h has a relatively high glass transition temperature (Tg) with high tensile strength and modulus,15, 16 and that the Diels–Alder polymerization product of furfuryl ester-terminated butylene succinate oligomer and BMIM has flexural strength properties superior to poly(butylene succinate).17 In this study, biseugenyl succinate (BEUS) and bis(4-maleimidephenyl) succinate (BMIS) were synthesized using succinic acid (SA) and eugenol (EU) to develop bio-based thermosetting bismaleimide resins with a higher level of performance than conventional bio-based polymers. Although SA is currently produced using petrochemicals, it can be produced naturally by many microorganisms as an intermediate in the central metabolism reactions or as a fermentation end-product. Bio-based SA is becoming increasing prominent, as it could provide a cost-effective and ecologically sustainable alternative to petrochemicals.18, 19 Eugenol (4-allyl-2-methoxyphenol) is a main component (80 wt%) of clove oil, which is produced mainly in Indonesia. Clove oil is widely used in perfumes, antioxidants, drugs, foods and as a flavoring agent.20, 21 In this study, the thermal and mechanical properties of the BEUS/BMIS and DABA/BMIM resins cured at 230 °C for 1 h are compared, and the difference in curing mechanism is elucidated by using FT-IR spectroscopy.
Experimental procedure
Materials
Succinic acid, succinyl chloride and EU were purchased from Kanto Chemical (Tokyo, Japan). Triethylamine, 4-dimethylaminopyridine and 4,4′-bismaleimidediphenylmethane (BMIM) were purchased from Tokyo Kasei Kogyo (Tokyo, Japan). 2,2′-Diallyl bisphenol A (DABA, technical grade, purity 85%) and N,N′-diisopropylcarbodiimide were purchased from Sigma-Aldrich Japan (Tokyo, Japan). Other reagents were commercially available with guaranteed purity and were used without further purification. p-Hydroxyphenylmalemide was synthesized as described in the literature.22
Synthesis of BEUS
To a solution of SA (11.9 g, 100 mmol) in 500 ml of dichloromethane EU (123 ml, 800 mmol), N,N′-diisopropylcarbodiimide (61.9 ml, 400 mmol) and 4-dimethylaminopyridine (24.4 g, 200 mmol) were added in this order, within several minutes. After stirring at 45 °C for 24 h, the reaction mixture was filtered, concentrated in vacuo and then poured into 400 ml of ethanol. The precipitate that was formed was collected by filtration, washed with ethanol and dried at 65 °C in a vacuum oven to give BEUS (13.8 g) as a white powder with a 34% yield: Proton nuclear magnetic resonance in DMSO-d6: δ 6.98 (m, 4H, Ha,b), 6.79 (d, 2H, Hc), 5.99 (m, 2H, CH2CH=CH2), 5.07 (m, 4H, CH2CH=CH2 ), 3.72 (s, 6H, OCH3), 3.36 (m, 4H, CH2CH=CH2) and 2.92 p.p.m. (s, 4H, -CH2CH2-); FTIR (KBr) 3073, 3007, 2973, 2933, 2838, 1751, 1636, 1602, 1506, 1461, 1361, 1289, 1262, 1189, 1139, 1027, 989, 906, 859, 807, 749 cm−1.
Synthesis of BMIS
To a solution of succinyl chloride (15.5 g, 0.100 mol) in tetrahydrofuran (250 ml), a solution of p-hydroxyphenylmalemide (38.0 g, 0.200 mol) and triethylamine (20.2 g, 0.200 mol) in tetrahydrofuran (250 ml) was added dropwise. After stirring for 24 h at room temperature, the reaction mixture was filtered, concentrated in vacuo and then poured into 500 ml of ethanol. The precipitate that was formed was collected by filtration, washed with ethanol and dried at 65 °C in a vacuum oven to give BMIS (7.68 g) as a yellow powder with a yield of 17%. Proton nuclear magnetic resonance in DMSO-d6: δ 7.40 (d, 4H, Ha,b), 7.27 (d, 4H, Hc,d), 7.19 (s, 4H, maleimide) and 3.00 p.p.m. (s, 4H, -CH2CH2-); FTIR (KBr) 3100, 1751, 1710, 1508, 1401, 1214, 1195, 1150, 829 and 702 cm−1.
Preparation of the cured materials of BEUS and BMIS
A mixture of BEUS (0.820 g, 2.00 mmol) and BMIS (0.921 g, 2.00 mmol) was stirred at 180–200 °C for 30 min to give a gelatinous material. The prepolymer that was obtained was compression-molded at 230 °C and 2 MPa for 1 h to produce a cured yellowish-brown sheet of BEUS and BMIS at a molar ratio of 1:1 (BEUS/BMIS 1/1). In a similar manner as BEUS/BMIS 1/1 was prepared, cured materials of BEUS and BMIS at molar ratios of 1:2 and 1: 3 (BEUS/BMIS 1/2 and BEUS/BMIS 1/3) were prepared.
Preparation of the cured materials of DABA and BMIM
A mixture of DABA (0.723 g, 2.00 mmol) and BMIM (0.719 g, 2.00 mmol) was stirred at 180–200 °C for 30 min to yield a gelatinous material. The prepolymer that was obtained was compression-molded at 230 °C and 2 MPa for 1 h to give a cured yellowish-brown sheet of DABA and BMIM at a molar ratio of 1:1 (DABA/BMIM 1/1). In a similar manner as DABA/BMIM 1/1 was prepared, cured materials of DABA and BMIM at molar ratios of 1:2 and 1:3 (DABA/BMIM 1/2 and DABA/BMIM 1/3) were prepared.
Measurements
Proton nuclear magnetic resonance spectra were recorded on a Bruker AV-400 (400 MHz) (Madison, WI, USA) using DMSO-d6 as a solvent. FTIR spectra were measured on a FTIR 8100 spectrometer (Shimadzu, Kyoto, Japan) by the KBr and attenuated total reflectance methods for the monomers and cured materials, respectively. The temperature at which 5% weight loss occurred was measured on a thermogravimetric analyzer TGA7 (Perkin–Elmer Japan, Yokohama, Japan) in a nitrogen atmosphere at a heating rate of 20 °C min−1. Dynamic mechanical analysis of the rectangular plates (length 30 mm, width 5 mm, thickness 0.5 mm) was performed on a Rheolograph Solid instrument (Toyo Seiki, Tokyo, Japan) under air atmosphere with a chuck distance of 20 mm, a frequency of 1 Hz and a heating rate of 2 °C min−1, based on ISO 6721-4:1994 (Plastics-Determination of dynamic mechanical properties, Part 4: Tensile vibration–non-resonance method). Thermomechanical analysis (TMA) was conducted on a TMA 2000S (MAC Science, Yokohama, Japan) at a heating rate of 10 °C min−1 in a nitrogen atmosphere. Tensile strength tests of the rectangular plates (length 35 mm, width 5 mm, thickness 0.5 mm) were performed at 25 °C using an Autograph AG-I (Shimadzu), based on the standard method for testing the tensile properties of plastics (JIS K7113-1995). The span length was 20 mm and the testing speed was 10 mm min−1. Five composite specimens were tested for each set of samples, and the mean values and the s.d. were calculated.
Results and discussion
Characterization of BEUS and BMIS
BEUS and BMIS (dially and bismalemimide compounds possessing succinate moieties) were synthesized from the reaction of SA with EU in the presence of N,N′-diisopropylcarbodiimide/4-dimethylaminopyridine and the reaction of succinyl chloride with p-hydroxyphenylmalemide in the presence of triethylamine, respectively, as shown in Figure 2. Figure 3 shows the Proton nuclear magnetic resonance spectra of the resulting BEUS and BMIS in DMSO-d6. The 1H signals of the allyl group and the succinate moiety for BEUS were observed at 5.99 (m, 2H), 5.07 (m, 4H) and 3.36 p.p.m. (m, 4H), and 2.92 p.p.m. (s, 4H), respectively. The 1H signals of the maleimide group and the succinate moiety for BMIS were observed at 7.19 (s, 4H) and 3.00 p.p.m. (s, 4H), respectively. The incorporation of biseugenyl and bismaleimide moieties in BEUS and BMIS was confirmed from the integral ratios of the 1H signals of the allyl and maleimide groups to the 1H signals of the succinate moieties, respectively.
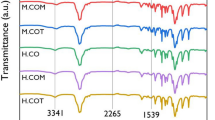
Figure 4 shows the FTIR spectra of BEUS and BMIS. In the spectrum of BEUS, the absorption peak ascribed to the ester C=O stretching vibration was observed at 1751 cm−1, and the benzene framework absorption peaks were observed at 1602 and 1506 cm−1. The peak formed due to the allyl C=C stretching vibration was observed at 1636 cm−1 and the peaks formed due to the allyl =CH out-of-plane bending vibration were observed at 989 and 906 cm−1. In the case of BMIS, the peaks formed due to ester C=O and imide C=O stretching vibrations were observed at 1751 and 1710 cm−1, respectively. Moreover, the benzene framework absorption peak was observed at 1508 cm−1. The peaks formed due to maleimide =CH out-of-plane bending vibration were observed at 829 and 702 cm−1. The changes in the absorption peaks characteristic of the allyl group, the maleimide group and the benzene ring of BEUS and BMIS were used for the evaluation of the curing mechanism of BEUS/BMIS.
Curing reactions of BEUS/BMIS and DABA/BMIM
The mixtures of BEUS and BMIS at the molar ratios of 1/1, 1/2 and 1/3 prepolymerized at 180–200 °C for 30 min were cured at 230 °C for 1 h to produce BEUS/BMISs 1/1, 1/2 and 1/3, respectively. The corresponding DABA/BMIM-cured products were also prepared under the same curing condition. Regarding the reaction mechanism for DABA/BMIM, Enoki et al.23, 24, 25 and Reyx et al.26 reported that the thermal reaction of the monofunctional model compounds, 2-allylphenol and N-phenylmaleimide (PMI), produced the ene-adduct, which underwent the Diels–Alder reaction twice with two molars of PMI to form the 3:1 adduct (Figure 5). In continued work, Shibahara et al.27 reported that the thermal reaction of DABA and PMI produced ene-adduct and chain-polymerized products, whereas the Diels–Alder adduct could not be detected. This difference in reactivity for 2-allylphenol/PMI and DABA/PMI was attributed to steric repulsion of DABA. Subsequently, Morgan et al.13, 14 reported that the curing mechanism of DABA/BMIM consisted of the ene addition reaction of the ally group to maleimide one, subsequent chain polymerization of maleimide and propenyl groups generated by the ene reaction and etherification by the dehydration reaction of the phenol moieties, based on the FTIR analysis of the reaction of DABA and BMI (Figure 6). The experimental result from remote fiber optic near-infrared spectroscopy by Mijoviæ and Andjeliæ28 also supported the occurrence of the etherification reaction, but a chemical structure was not proposed. Such an etherification reaction of aromatic hydroxy compounds is generally difficult to achieve by an ionic mechanism. However, it is well known that the oxidative coupling polymerization of 2,6-dimethylphenol in the presence of oxygen and an oxidizing catalyst (Cu2+/amine) gives poly(2,6-dimethylphenylene ether) and water. A similar oxidative coupling reaction of phenol moieties induced by a charge transfer from a donor (DABA) to an acceptor (BMIM) may possibly occur in DABA/BMIM. To confirm their curing mechanism of DABA/BMIM, we measured the FTIR spectra of DABA/BMIMs 1/1, 1/2 and 1/3. In agreement with the reported mechanism, the disappearance of the absorption peaks ascribed to the allyl group (1634, 991, 907 cm−1) and to the maleimide group (840 and 718 cm−1) was confirmed (Figure 7). The appearance of the absorption peak of the propenyl =CH out-of-plane bending vibration at 970 cm−1 for DABA/BMIM suggests the occurrence of the ene reaction of the allyl group with the maleimide group. The fact that the benzene framework absorption peaks (1508 and 1493 cm−1) observed for BMIM and DABA are retained for DABA/BMI indicates that the Diels–Alder reaction has not occurred after the ene reaction. Furthermore, an increase of ether C-O-C anti-symmetrical stretching at approximately 1180 cm−1 suggests the occurrence of etherification.
Although the absorption peaks (1636, 989 and 906 cm−1) characteristic of the allyl group, and the absorption peaks (829 and 702 cm−1) characteristic of the CH=CH of the maleimide group, observed for BEUS and BMIS, are considerably diminished for BEUS/BMIS, the peaks remained to some extent (Figure 4). Moreover, the absorption peaks (1602, 1508 and 1506 cm−1), characteristic of the benzene framework, observed for BMIS and BEUS were retained for BEUS/BMISs 1/1, 1/2 and 1/3. The absorption peak characteristic of the propenyl group at 970 cm−1 observed for DABA/BMIM was not observed for BEUS/BMIS, suggesting that there was no occurrence of the ene reaction. Furthermore, the formation of new ether groups observed for DABA/BMIM was not observed for BEUS/BMIS because BEUS has no phenolic hydroxy group. The reaction mechanism deduced from the FTIR spectral data for BEUS/BMIS is shown in Figure 8. Chain homo- and copolymerization of the allyl and maleimide groups of BEUS and BMIS proceeded smoothly for all the BEUS/BMIS mixtures. This reaction mechanism is in agreement with the monofunctional model reactions reported by Enoki et al.,23, 24 where chain copolymerization occurs in the reaction of PMI with allylbenzene derivatives, such as 4-allylanisole and allylbenzoate, in contrast to the progress of the ene reaction of PMI with allylphenol derivatives, such as 2-allylphenol and EU. The occurrence of the ene reaction in the latter combination may be related to the thermal-induced charge transfer from allylphenol to phenylmaleimide.
Thermal and mechanical properties of BEUS/BMIS and DABA/BMIM
Figure 9 shows dynamic mechanical analysis curves of BEUS/BMIS and DABA/BMIM cured at 230 °C for 1 h. The tan δ peak temperature corresponding to Tg and storage modulus (E′) for BEUS/BMIS increased with increasing BMIS content (Table 1). All the BEUS/BMISs did not have a rubbery plateau region of E′, suggesting that crosslinking density is not so high. The E′ of DABA/BMIM decreased with temperature, and then started increasing at the temperature higher than the curing temperature of 230 °C, indicating that DABA/BMIM is not fully cured at 230 °C for 1 h. The curing at a higher temperature is necessary for the addition polymerization of the propenyl moiety produced by the ene reaction. When the tan δ peak temperature of the samples cured at 230 °C for 1 h were compared, BEUS/BMISs 1/2 and 1/3 had higher values than DABA/BMIMs 1/2 and 1/3, respectively. DABA/BMIMs 1/1 and 1/1.2 show Tg values of 295 and 310 °C when cured at 180 °C/1 h+200 °C/2 h+250 °C/6 h.5, 8 Although DABA/BMIM has a higher Tg than BEUS/BMIS in a fully cured state, BEUS/BMIS is more suitable for the curing at a lower temperature and a shorter time than DABA/BMIM.
Table 1 summarizes the Tg values measured by TMA and 5% weight loss temperature measured by TGA for BEUS/BMIS and DABA/BMIM. Although the Tg determined by TMA was lower than the tan δ peak temperature by dynamic mechanical analysis, the data from TMA showed a trend similar to the data from dynamic mechanical analysis. The 5% weight loss temperature of BEUS/BMIS was lower than the 5% weight loss temperature of DABA/BMIM, suggesting that the succinate moieties of BEUS and BMIS are more unstable thermally than the diphenylmethane and diphenylpropane moieties of DABA and BMIM.
Figure 10 shows the tensile properties at 20 °C for BEUS/BMIS and DABA/BMIM cured at 230 °C for 1 h. DABA/BMIM 1/1 shows a tensile strength of 85 MPa, a tensile modulus of 4430 MPa and elongation of 2.3%, when cured at 180 °C/1 h+200 °C/2 h+250 °C/6 h.9, 12 The tensile strength and modulus of the fully cured DABA/BMIM 1/1 are higher than the comparable values of BEUS/BMISs 1/1, 1/2 and 1/3 cured at 230 °C/1 h. When the tensile properties of the materials cured at 230 °C for 1 h are compared, the BEUS/BMISs 1/1, 1/2 and 1/3 had higher tensile strength than the DABA/BMIMs 1/1, 1/2 and 1/3.
Conclusions
Bio-based diallyl and bismalemide compounds, BEUS and BMIS were synthesized by using EU and SA. A gelled mixture of BEUS and BMIS in a molar ratio of 1/1, 1/2 or 1/3 at 200 °C for 0.5 h was compression-molded at 230 °C for 1 h to give a cured BEUS/BMIS product. The properties of BEUS/BMIS were compared with the properties of DABA/BMIM cured under the same conditions. The FTIR analysis of the cured materials revealed that chain polymerization of the allyl and maleimide groups occurs for BEUS/BMIS, whereas a stepwise ene reaction and subsequent chain polymerization in addition to etherification occur for DABA/BMIM. The Tg and tensile strength of BEUS/BMIS 1/2 and 1/3 were higher than the comparable parameters for DABA/BMIM 1/2 and 1/3, respectively. Although a fully cured DABA/BMIM under more severe conditions shows higher thermal and mechanical properties, BEUS/BMIS is a high-performance bio-based thermosetting resin curable at a lower temperature and shorter time than DABA/BMIM.
References
Kaplan, D. L. In Biopolymers from Renewable Resources (Kaplan, D.L., ed) Ch.1, 1–26 (Springer, 1998).
Mohanty, A. K., Misra, M. & Hinrichsen, G. Biofibres, biodegradable polymers, biocomposites: an overview. Macromol. Mater. Engn. 276, 1–24 (2000).
Mohanty, A. K., Misra, M. & Drzal, L. T. Sustainable bio-composites from renewable resources: opportunities and challenges in the green materials world. J. Polym. Environ. 10, 19–26 (2002).
Kimura, Y. Molecular, structural, and material design of bio-based polymers. Polym. J. 41, 797–807 (2009).
Raquez, J.- M., Deléglise, M., Lacrampe, M.- F. & Krawczak, P. Thermosetting (bio)materials derived from renewable resources: a critical review. Prog. Polym. Sci. 35, 487–509 (2010).
Nemoto, T., Konishi, G., Tojo, Y. & Funaoka, M. Development of lignin as a transparent resin: evaluation of thermal and optical properties of alkoxylated lignophenols. Polym. J. 42, 896–900 (2010).
Murakami, S., Aoki, N. & Matsumura, S. Bio-based biodegradable hydrogels prepared by crosslinking of microbial poly(γ-glutamic acid) with L-lysine in aqueous solution. Polym. J. 43, 414–420 (2011).
Tsujimoto, T., Haza, Y., Yin, Y. & Uyama, H. Synthesis of branched poly(lactic acid) bearing a castor oil core and its plasticization effect on poly(lactic acid). Polym. J. 43, 425–430 (2011).
Nair, C. P. R. Advances in addition-cure phenolic resins. Prog. Polym. Sci. 29, 401–498 (2004).
Hopewell, J. L., George, G. A. & Hill, D. J. T. Quantitative analysis of bismaleimide-diamine thermosets using near infrared spectroscopy. Polymer 41, 8221–8229 (2000).
Chaplin, A., Hamerton, I., Herman, H., Mudhar, A. K. & Shaw, S. J. Studying water uptake effect in resins based on cyanate ester/bismalemide blends. Polymer 41, 3945–3956 (2000).
King, J. J., Chaudhari, M. & Zahir, S. A new bismaleimide system for high performance applications. 29th SAMPE Symp. 29, 392–403 (1984).
Morgan, R. J., Shin, E. E., Rosenberg, B. & Jurek, A. Characterization of the cure reactions of bismaleimide composite matrices. Polymer 38, 639–646 (1997).
Rozenberg, B. A., Dzhavadyan, E. A., Morgan, R. & Shin, E. High-performance bismaleimide matrices: cure kinetics and mechanism. Polym. Adv. Technol. 13, 837–844 (2002).
Shibata, M., Teramoto, N. & Nakamura, Y. High performance bio-based thermosetting resins composed of tung oil and bismaleimide. J. Appl. Polym. Sci. 119, 896–901 (2011).
Hirayama, K., Irie, T., Teramoto, N. & Shibata, M. High performance bio-based thermosetting resins composed of dehydrated castor oil and bismaleimide. J. Appl. Polym. Sci. 114, 1033–1039 (2009).
Shibata, M., Teramoto, N., Akiba, T. & Ogihara, M. High-performance hybrid materials prepared by the thermo-reversible Diels-Alder polymerization of furfuryl ester-terminated butylene succinate oligomers and maleimide compounds. Polym. J. 43, 455–463 (2011).
Zheng, P., Dong, J.- J., Sun, Z.- H. & Fang, L. Fermentative production of succinic acid from straw hydrolysate by Actinobacillus succinogenes. Biores. Technol. 100, 2425–2429 (2009).
Raab, A. M., Gebhardt, G., Bolotina, N., Weuster-Botz, D. & Lang, C. Metabolic engineering of Saccharomyces cerevisiae for the biotechnological production of succinic acid. Metabolic Engn. 12, 518–525 (2010).
Sabaa, M. W. & Mohamed, R. R. Organic thermal stabilizers for rigid poly(vinyl chloride). Part XIII: Eugenol (4-allyl-2-methopxy-phenol). Polym. Degrad. Stabl. 92, 587–595 (2007).
Peppas, N. A. & Ende, D. J. A. Controlled release of perfumes from polymers. II. Incorporation and release of essential oils from glassy polymers. J. Appl. Polym. Sci. 66, 509–513 (1997).
Wu, C. S., Liu, Y. L. & Hsu, K. Y. Maleimide-epoxy resins: preparation, thermal properties, and flame retardance. Polymer 44, 565–573 (2003).
Enoki, T., Takeda, T. & Ishii, K. Effects of catalysts on the model reactions of several maleimide resin systems (III): ally-modified maleimide. High Perform. Polym. 7, 399–410 (1995).
Enoki, T., Okubo, H., Takeda, T. & Ishii, K. [Investigaton on the reaction between N-phenylmaleimide and ally compounds]. Netsukoukasei Jushi 62, 26–30 (1995).
Enoki, T., Okubo, H., Ishii, K. & Shibahara, S. [Curing reactions of maleimide resins. IV. Effects of catalysts on the reactions of N-phenylmaleimide/allyl compound systems]. Netsukoukasei Jushi 12, 9–18 (1991).
Reyx, D., Campistron, I., Caillaud, C., Villatte, M. & Cavedon, A. Thermal reactions between N-phenyl maleimide and 2-allylphenol as a model for crosslinking reaction in bismaleimide polymerization with diallylbisphenol-A. Makromol. Chem. Phys. 196, 775–785 (1995).
Shibahara, S., Yamamoto, T., Yamaji, T., Motoyoshiya, J. & Hayashi, S. Thermal reactions of N-phenylmaleimide and mono- or di-functional allylphenols. Polym. J. 30, 404–409 (1998).
Mijoviæ, J. & Andjeliæ, S. Study of the mechanism and rate of bismaleimide cure by remote in situ real time fiber optic near-infrared spectroscopy. Macromolecules 29, 239–246 (1996).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shibata, M., Teramoto, N., Shimasaki, T. et al. High-performance bio-based bismaleimide resins using succinic acid and eugenol. Polym J 43, 916–922 (2011). https://doi.org/10.1038/pj.2011.87
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pj.2011.87
Keywords
This article is cited by
-
Eugenol: extraction, properties and its applications on incorporation with polymers and resins—a review
Polymer Bulletin (2023)
-
Preparation and mechanism of shape memory bismaleimide resins with high transition temperature, high toughness and good processability
Journal of Materials Science (2018)
-
High performance thermosets based on multifunctional intermediates containing allyl, maleimide and benzoxazine groups
Journal of Polymer Research (2013)