Abstract
Organic–inorganic hybrids containing [Ti4(μ3-O)(OiPr)5(μ-OiPr)3(O3PPh)3]·thf as element-blocks were prepared by hybridization with poly(dimethylsiloxane), poly(methylsilsesquioxane), poly(ethoxysilsesquioxane), poly(methyl methacrylate), poly(vinyl alcohol), poly(4-vinylphenol), poly(styrene-co-allyl alcohol) or poly(bisphenol A-co-epichlorohydrin). The concentration of the titanium cluster was increased to 40 wt% to form crack-free films with high transparency. The temperature at which 10 wt% weight loss occurred increased with the concentration of the titanium cluster because an alcohol exchange reaction was expected between the titanium cluster and polymers. The mechanical strengths and strains of poly(dimethylsiloxane) hybrids were very low. The tensile strengths and elongations of poly(methyl methacrylate) hybrids increased with the increase in the titanium cluster concentration. The tensile strengths and elongations of poly(vinyl alcohol) hybrids were highest when the titanium cluster concentration was 10 wt%.
Similar content being viewed by others
Introduction
Organic–inorganic hybrids containing element-blocks, a structural unit consisting of various groups of elements, are promising materials by virtue of their strong performance characteristics, such as high mechanical strength, thermal stability, gas permeability, light emission and electron conductivity.1 These characteristics appeared by the hybridization of organic polymer with inorganic material. Indeed, organic–inorganic hybrid materials containing polyhedral octasilsesquioxanes are reported to show strong gas separation2 and refractive index3 qualities.
Other cage-type compounds, such as titanium phosphonate clusters, are used as inorganic components in next-generation organic–inorganic hybrids. Titanium phosphonate clusters are composed of phospha-titanoxane linkages (Ti-O-P bonds) and cage structures. We previously reported on organic–inorganic hybrids using poly(vinyl alcohol) (PVA) or poly(methyl methacrylate) (PMMA) with [Ti4(μ3-O)(OiPr)5(μ-OiPr)3(O3PPh)3]·thf (TiOPPh).4 A hybrid of PVA containing TiOPPh was composed by the alcohol exchange reaction of PVA with isopropyl groups in TiOPPh. However, PVA has the disadvantage of low miscibility in organic solvents (dimethylformamide and dimethyl sulfoxide) in the preparation of organic–inorganic hybrid materials. Other candidates that are miscible in common organic solvents include silicone polymers such as poly(dimethylsiloxane) (PDMS), poly(methylsilsesquioxane) (PMS) and poly(ethoxysilsesquioxane) (PEOS), as well as organic polymers such as poly(4-vinylphenol) (PVP), poly(styrene-co-allyl alcohol) (PSA) and poly(bisphenol A-co-epichlorohydrin) (PBE), as shown in Scheme 1. In this work, hybrids containing TiOPPh were prepared using silicone polymers and organic polymers. In addition, mechanical properties of hybrids of PDMS, PMMA and PVA containing TiOPPh were discussed.
Experimental Procedures
Measurements
Nuclear magnetic resonance (NMR) spectra were recorded using a JEOL Resonance JNM-ECP 300 Spectrometer (JEOL, Akishima, Japan) (1H at 300.53 MHz and 29Si at 59.70 MHz) at 24 °C. The chemical shifts were reported in parts per million (p.p.m.) relative to chloroform-d (CDCl3) used as an internal standard (for 1H: 7.26 p.p.m. in residual chloroform) and tetramethylsilane as an internal standard (for 29Si: 0 .00 p.p.m.). Gel permeation chromatography was performed using a high-performance liquid chromatography system (LC-6AD; Shimadzu, Kyoto, Japan) attached to a Polymer Laboratory gel 5 μm Mixed-D column. Tetrahydrofuran was used as the eluent (1 ml min−1). RID-10A was used as the detector. The molecular weight was calculated based on polystyrene standards. Fourier transform infrared (IR) spectra were recorded on a Fourier Transform Infrared Spectrophotometer (FT/IR-6100; JASCO, Hachioji, Japan) using an attenuated total reflectance (ZnSe prism, JASCO ATR PRO 0450-S), or KBr pellet, coating on silicon wafer. Thermogravimetric-differential thermal analysis was performed using a Thermogravimetric-Differential Thermal Analysis Analyzer (2000SE; Netzsch Japan, Yokohama, Japan). The samples were heated to 1000 °C under an airflow at the rate of 10 °C min−1. Transmittance spectra were recorded using a JASCO V-670 Spectrophotometer (JASCO, Hachioji, Japan) equipped with an Integrating-sphere Photometer (JASCO ISN-470 type; JASCO) in the 200–800 nm wavelength range. The tensile strength was recorded using a Shimadzu Autograph AG-50 kN Xplus (Shimadzu, Kyoto, Japan) at the rate of 2 mm min−1. The size of the test samples is shown below: height 25 mm, width 5 mm and thickness 50–100 μm.
Materials
All solvents were purified by a standard process5 and stored over activated molecular sieves. PVA (degree of polymerization, 500) and tetraethylammonium hydroxide (20% aqueous solution) were purchased from Wako Pure Chemical Industries (Tokyo, Japan). Tetraethylammonium hydroxide was concentrated by removal of the water under vacuum. PVP (Mw=25 000 g mol−1), PSA (Mw=2200 g mol−1, allyl alcohol 40 mol%), PBE (Mw=40 000 g mol−1) and PMMA (Mw=997 000 g mol−1) were purchased from Sigma-Aldrich (Tokyo, Japan) and used as received. Trimethoxy(methyl)silane, tetraethoxysilane (TEOS) and chloro(trimethyl)silane were purchased from Tokyo Chemical Industry (Tokyo, Japan) and purified by distillation before use. Octamethylcyclotetrasiloxane was purchased from Shin-Etsu Chemical Co. (Tokyo, Japan) and purified by distillation before use. TiOPPh was prepared as described previously.4
Synthesis
Preparation of PDMS
Octamethylcyclotetrasiloxane (30.0 g, 0.10 mol) and tetraethylammonium hydroxide (0.60 g, 4 mmol) were placed into a 100 ml four-necked flask equipped with an Allihn condenser and a mechanical stirrer. The mixture was stirred at 100 °C for 3 h at 200 r.p.m. The viscous liquid was extracted with CH2Cl2. The organic layer was washed with brine, and dried with MgSO4. After filtration, the solution was concentrated to remove vapor compounds under vacuum at 60 °C. PDMS was obtained as a colorless viscous liquid (27.2 g, 91%).
1H NMR (300 MHz, CDCl3 per p.p.m.) δ 0.08 (brs, Si-CH3). 29Si NMR (60 MHz, CDCl3 per p.p.m.) δ −21.55. Mw=93 300 g mol−1, Mw/Mn=1.9.
Preparation of PMS
Trimethoxy(methyl)silane (27.2 g, 0.20 mol) and MeOH (13.3 g, 0.42 mol) were placed into a 200 ml four-necked flask equipped with nitrogen inlet and outlet tubes and a mechanical stirrer. The mixture was then cooled in an ice bath for 10 min. Water and hydrochloric acid (molar ratios; H2O/trimethoxy(methyl)silane=1.0, HCl/trimethoxy(methyl)silane=0.105) were added. The mixture was stirred in an ice bath for 10 min and then at room temperature for 10 min, followed by heating at 70 °C for 3 h at 150 r.p.m. with a 360 ml min−1 nitrogen flow. PMS was obtained as colorless liquid (16.3 g).
1H NMR (300 MHz, CDCl3 per p.p.m.) δ 0.12 (brs, Si-CH3), 3.51 (brs, Si-OCH3). 29Si NMR (60 MHz, CDCl3 per p.p.m.) δ −49.35 (T1, content ratio of 6%), −58.87 (T2, that of 50%) and −67.17 (T3, that of 44%). Mw=3800 g mol−1, Mw/Mn=1.8.
Preparation of PEOS
TEOS (34.8 g, 0.17 mol) and EtOH (15.9 g, 0.35 mol) were placed into a 300 ml four-necked flask equipped with nitrogen inlet and outlet tubes and a mechanical stirrer. The mixture was then cooled in an ice bath for 10 min. Water and hydrochloric acid (molar ratios; H2O/TEOS=1.7, HCl/TEOS=0.105) were added. The mixture was stirred in an ice bath for 10 min and then at room temperature for 10 min, followed by heating at 80 °C for 2 h at 150 r.p.m. with a 360 ml min−1 nitrogen flow. The resulting product was dissolved into 40 ml tetrahydrofuran (THF), then 2 ml TMSCl was added, followed by stirring for 1 day. The mixture was concentrated to remove vapor compounds, and PEOS was obtained (15.7 g).
1H NMR (300 MHz, CDCl3 per p.p.m.) δ 0.16 (brs, Si-CH3), 1.25 (brs, Si-OCH2CH3) and 3.89 (brs, Si-OCH2CH3). 29Si NMR (60 MHz, CDCl3 per p.p.m.) δ 12.76 (M, content ratio of 8%), −95.86 (Q2, that of 12%) and −102.78 (Q3, that of 80%). Mw=7200 g mol−1, Mw/Mn=2.7.
Preparation of free-standing films
Polymer solution of THF or toluene was added to TiOPPh and stirred for 3 h at room temperature. The mixture was poured into a 50 mm φ Teflon Petri dish followed by curing at 50 °C for 1 day and then at 120 °C for 1 day.
Preparation of PVP/TiOPPh hybrid thin films
The solution of PVP (0.125 g) dissolved in THF (5 ml) was added to TiOPPh, and stirred for 3 h at room temperature. Hybrid thin films were prepared by spin coating the solution on a silicon wafer (700 r.p.m., 30 s) and then heating at 120 °C for 2 min in air, repeated for a total of three times.
Results and discussion
Preparation and properties of silicone polymers/TiOPPh hybrid
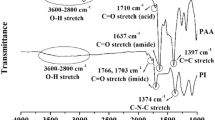
The silicone/TiOPPh hybrids were prepared by mixing silicone polymers with TiOPPh in toluene (Table 1). Free-standing PDMS/TiOPPh films were prepared in the concentration of 20–40 wt% TiOPPh, and the obtained films were highly flexibile. When the concentration was <20 wt%, the hybrids were oily, viscous and adhesive. On the other hand, when the concentration was >40 wt%, the hybrids were obtained as glassy solids. In the IR spectra of the hybrids, no absorption band assigned to νSi-O-Ti (around 950 cm−1)6 was observed. The transparency of PDMS hybrid was >89% (at 420 nm) even at a concentration of 40 wt%. This transparency is higher than the PMMA composite (43% at 420 nm, due to the aggregation of TiOPPh in PMMA).4 The hybrids using PDMS were, therefore, composed of segregated polymer domains with uniform dispersions of TiOPPh in PDMS. The thermogravimetric traces for PDMS hybrids are shown in Figure 1(i) and Table 2. The temperatures at which 10% weight loss occurred (Td10) were 414 °C (PDMS), 281 °C (TiOPPh 20 wt%) and 264 °C (TiOPPh 40 wt%). The decrease of Td10 was very similar to the tendency of PDMS/metal oxide composite that was reported to accelerate the oxidation of methyl groups and the depolymerization with formation of cyclosiloxanes.7 The char yield of PDMS only and TiOPPh only at 1000 °C in air were 28.2% and 40.4%, respectively. Thus, the thermal decomposition of PDMS mainly occurs by the depolymerization because the char yield would be 81.0% if the thermal decomposition of PDMS was proceed to oxidize all of the methyl groups. The char yield of PDMS/20 wt%, 30 wt% and 40 wt% TiOPPh were 28.9%, 31.2% and 37.2%, respectively, where we can expect 30.6% (for 20 wt%), 31.9% (for 30 wt%) and 33.1% (for 40 wt%). The depolymerization of PDMS would be more accelerated when the content of TiOPPh is low, and the oxidation of PDMS would be slightly accelerated at high concentration.
Thermogravimetric analyses of (i) PDMS (PDMS (a), TiOPPh 20 wt% (b) and TiOPPh 40 wt% (c)), (ii) PMS (PMS (a), TiOPPh 20 wt% (b) and TiOPPh 40 wt% (c)) and (iii) PEOS hybrids (PEOS (a) and TiOPPh 20 wt% (b)). PDMS, poly(dimethylsiloxane); PEOS, poly(ethoxysilsesquioxane); PMS, poly(methylsilsesquioxane); TiOPPh, [Ti4(μ3-O)(OiPr)5(μ-OiPr)3(O3PPh)3]·thf. A full color version of this figure is available at the Polymer Journal journal online.
The free-standing PMS/TiOPPh films were prepared in the concentration of 10–40 wt% TiOPPh, but the films were brittle and easily broken. The PMS hybrid containing 40 wt% TiOPPh was yellow and 67% transparent at 420 nm. Other films were colorless with 93% transparent at 420 nm. In the IR spectra of 40 wt%, a new small absorption band was observed at 912 cm−1, which was assigned to νSi–O–Ti. Unfortunately, this band was so weak that this band was not observed when the concentration was <40 wt%. The Td10, as shown in Figure 1(ii), decreased when the concentration of TiOPPh was increased as 573 °C for PMS, 557 °C for 20 wt% TiOPPh and 452 °C for 40 wt% TiOPPh. TiOPPh might accelerate the oxidation of methyl group on PMS as with thermal decomposition of PDMS.
Free-standing PEOS hybrid films were barely formed because the hybrids were rigid and brittle. In the IR spectrum of the hybrid containing 40 wt% TiOPPh, a new absorption band assigned to νSi-O-Ti (at 908 cm−1) was observed. The Td10 of the PEOS hybrid decreased slightly compared to that of only PEOS (Figure 1 (iii)). TiOPPh maybe act as a catalyst of hydrolytic polycondensation of alkoxysilane such as metal acetylacetonate complexes.8
Preparation and properties of organic polymers/TiOPPh hybrids
The organic polymers/TiOPPh hybrids were prepared by mixing organic polymers with TiOPPh in THF (Table 1). When TiOPPh was added to PVP solution, the solution immediately changed from colorless to red, and the coloration increased with the concentration of TiOPPh. This phenomenon was similar to that observed in the synthesis of [Ti2(μ-OPh)2(OPh)6(HOPh)2] by the reaction of Ti(O/Pr)4 with phenol.9 Thus, the alcohol exchange reaction is expected to proceed between PVP and TiOPPh. These free-standing films were rigid. The coating films were prepared on a silicon wafer by spin coating, which were colored in orange. The intensity of the hydroxyl group at 3200–3500 cm−1 in the Fourier transform IR spectra decreased with the increase in TiOPPh. The Td10, as shown in Figure 2(i), were 242 °C (PVP) and 406 °C (50 wt% concentration), showing that TiOPPh acts as a good crosslinker to PVP matrix.
Thermogravimetric analyses of (i) PVP (PVP (a), TiOPPh 20 wt% (b) and TiOPPh 50 wt% (c)), (ii) PSA (PSA (a), TiOPPh 20 wt% (b) and TiOPPh 40 wt% (c)) and (iii) PBE hybrids (PBE (a), TiOPPh 20 wt% (b) and TiOPPh 50 wt% (c)). PBE, poly(bisphenol A-co-epichlorohydrin); PSA, poly(styrene-co-allyl alcohol); PVP, poly(4-vinylphenol); TiOPPh, [Ti4(μ3-O)(OiPr)5(μ-OiPr)3(O3PPh)3]·thf. A full color version of this figure is available at the Polymer Journal journal online.
Free-standing PSA hybrid films were barely formed because of the low molecular weight (2200 g mol−1) of PSA. In the IR spectra, the intensity of the hydroxyl group decreased as the concentration of TiOPPh increased. The Td10 were 332 °C (PSA), 241 °C (PSA 20 wt%) and 279 °C (PSA 40 wt%) (Figure 2(ii)).
Free-standing PBE hybrid films were prepared at concentrations of <50 wt% TiOPPh, and the obtained films were orange. In the IR spectra of PBE hybrids, The intensity of νOH decreased with the increase in TiOPPh content. Thus, TiOPPh was reacted with hydroxyl groups of PBE. Also, the top of the absorption bands assigned to ether groups (νC–O–C) shifted from 1036 cm−1 (PBE) to 1032 cm−1 (PEB hybrids). In the case of Zn crown ether-type complex coordinated from the oxygen atoms to zinc atom, the band of νC–O–C was shifted to decrease 6 cm−1 less than only crown ether ligand.10 Therefore, the PBE hybrids suggest the formation of chelate by the coordination of the oxygen atom to titanium. The transmittance of free-standing films was 89% (PBE), 55% (10 wt% TiOPPh) and 40% (50 wt% TiOPPh) at 420 nm. The Td10 values were 397 °C (PBE), 350 °C (20 wt% TiOPPh) and 341 °C (50 wt% TiOPPh) (Figure 2(iii)). The thermal stabilities of the PSA and PBE hybrids were lower than those of PSA and PBE polymers and PVA hybrid as reported before.4
Tensile strengths of free-standing films
The measured tensile strength of free-standing films of PDMS and PVA hybrids, and PMMA composites are summarized in Figure 3 and Table 3. The free-standing PDMS hybrid films show very low tensile strengths: 0.6 MPa (PDMS 20 wt% TiOPPh) and 0.2 MPa (30 wt% TiOPPh). The tensile strengths of PDMS-TEOS hybrid materials containing a titanium crosslinker were reported to be increased by the increase in the titanium content.11 Moreover, the tensile strength of PDMS materials is known to depend strongly on the crosslinked network.12 Therefore, the molecular interaction between PDMS and TiOPPh would be weak.
Stress–strain curves of (i) PMMA (PMMA (a), TiOPPh 2.5 wt% (b) and TiOPPh 10 wt% (c)), and (ii) PVA (PVA (a), TiOPPh 2.5 wt% (b), TiOPPh 10 wt% (c), TiOPPh 20 wt% (d) and TiOPPh 30 wt% (e)). PMMA, poly(methyl methacrylate); PVA, poly(vinyl alcohol); TiOPPh, [Ti4(μ3-O)(OiPr)5(μ-OiPr)3(O3PPh)3]·thf. A full color version of this figure is available at the Polymer Journal journal online.
The tensile strengths of PMMA composite films are shown in Figure 3(i). Stress and strain increased with the increase in the concentration of TiOPPh for 0, 2.5 and 10 wt%. The tensile strength was increased about threefold from only PMMA film by mixing TiOPPh in 10 wt%. This improvement is higher than that in PMMA-montmorillonite composite material, which showed an improvement of ca. 1.1-fold.13 The strain was also increased with the increase in the TiOPPh concentration, suggesting mixing PMMA with TiOPPh will yield hard and brittle composites.
The tensile strengths of PVA hybrid films are shown in Figure 3(ii). The strain increased with the similar manner with PMMA hybrids to 10wt% cluster contain. On the other hand, the strain decreased when the content was increased from 20 and 30 wt%. The Young's modulus was evaluated for the stress and strain values of the initial stage. The value was increased by the TiOPPh content that suggests the formation of brittle and hard hybrids due to the crosslinking by TiOPPh. Maximum strain and stress were observed when the TiOPPh content was 10 wt% because TiOPPh acts as a good crosslinker to form hard and brittle hybrids.
Conclusion
Organic–inorganic hybrids containing TiOPPh as a new element block were prepared by using silicon polymers such as PDMS, PMS and PEOS and organic polymers such as PMMA, PVA, PVP, PSA and PBE. The concentration of TiOPPh was increased to 40 wt% to form a transparent film for PDMS, PMS, PVA and PBE, whereas the concentration was 20 wt% to form such a film for PMMA. The temperature at which the 10 wt% weight loss occurred was increased for the hybrid using PVP because the alcohol exchange reaction between TiOPPh and PVP was expected to form a rigid network, whereas the 10 wt% weight loss temperature decreased with the increase in TiOPPh concentration for PSA, PBE, PDMS, PMS and PEOS. The PDMS hybrids showed very low tensile strengths and elongations. The tensile strengths and elongations of PMMA hybrids increased with the increase in the TiOPPh concentration. The tensile strengths and elongations of PVA hybrid were highest when the concentration of TiOPPh was 10 wt%. As a result, TiOPPh was found to be a good crosslinker to form hard and brittle hybrids.

Chemical structures of polymers and [Ti4(μ3-O)(OiPr)5(μ-OiPr)3(O3PPh)3]·thf (TiOPPh).
References
Chujo, Y. & Tanaka, K. New polymer materials based on element-blocks. Bull. Chem. Soc. Jpn 88, 633–643 (2015).
Kanezashi, M., Shioda, T., Gunji, T. & Tsuru, T. Gas permeation properties of silica membranes with uniform pore sizes derived from polyhedral oligomeric silsesquioxane. AIChE J. 58, 1733–1743 (2012).
Tanaka, K., Yamane, H., Mitamura, K., Watase, S., Matsukawa, K. & Chujo, Y. Transformation of sulfur to organic–inorganic hybrids employed by networks and their application for the modulation of refractive indices. J. Polym. Sci. Part A 52, 2588–2595 (2014).
Hayami, R., Wada, K., Sagawa, T., Tsukada, S., Watase, S. & Gunji, T. Preparation and properties of organic–inorganic hybrid polymer films using [Ti4(μ3-O)(OiPr)5(μ-OiPr)3(O3PPh)3]·thf. Polym. J. 49, 223–228 (2017).
Armarego, W. L. F. & Chai, C. Purification of Laboratory Chemicals 7th edn (Elsevier, Oxford, UK, 2012).
Julián, B., Gervais, C., Cordoncillo, E., Escribano, P., Babonneau, F. & Sanchez, C. Synthesis and characterization of transparent PDMS-metal-oxo based organic–inorganic nanocomposites. Chem. Mater. 15, 3026–3034 (2003).
Gun'ko, V. M., Borysenko, M. V., Pissis, P., Spanoudaki, A., Shinyashiki, N., Sulim, I. Y., Kulik, T. V. & Palyanytsya, B. B. Polydimethylsiloxane at the interfaces of fumed silica and zirconia/fumed silica. Appl. Surf. Sci. 253, 7143–7156 (2007).
Adachi, K. & Hirano, T. Good linear relationship between logarithms of Eigen's water exchange constants for several divalent metal ions and activation energies of corresponding metal-catalyzed alkoxysilane hydrolysis in ethylene-propylene copolymer system. Eur. Polym. J. 44, 542–549 (2008).
Svetich, G. W. & Voge, A. A. The crystal and molecular structure of sym-trans-di-μ-phenoxyhexaphenoxydiphenolatodititanium (IV). Acta Crystallogr. B 28, 1760 (1972).
Logacheva, N. M., Baulin, V. E., Tsivadze, A. Y., Pyatova, E. N., Ivanova, I. S., Velikodny, Y. A. & Chernyshev, V. V. Ni (II), Co (II), Cu (II), Zn (II) and Na (I) complexes of a hybrid ligand 4′-(4′′′-benzo-15-crown-5)-methyloxy-2,2′:6′,2′′-terpyridine. Dalton Trans. 2009, 2482–2489 (2009).
Glaser, R. H. & Wilkes, G. L. Structure property behavior of polydimethylsiloxane and poly(tetramethylene oxide) modified TEOS based sol–gel materials V. Effect of titaniumisopropoxide incorporation. Polym. Bull. 19, 51–57 (1988).
Liu, M., Sun, J., Sun, Y., Bock, C. & Chen, Q. Thickness-dependent mechanical properties of polydimethylsiloxane membranes. J. Micromech. Microeng. 19, 035028 (2009).
Lee, D. C. & Jang, L. W. Preparation and characterization of PMMA-clay hybrid composite by emulsion polymerization. J. Appl. Polym. Sci. 61, 1117–1122 (1996).
Acknowledgements
This work was supported by a Grant-in-Aid for Scientific Research on Innovative Areas ‘New Polymeric Materials Based on Element-Blocks' (No 2401) (JSPS KAKENHI Grant Number JP24102008). This work was supported also by JSPS KAKENHI Grant Number JP16K17951. Shinji Ogihara, Ryuta Kitamura and Ryosuke Matsuzaki are greatly acknowledged for their technical assistance in the tensile strength measurement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Hayami, R., Wada, K., Nishikawa, I. et al. Preparation and properties of organic–inorganic hybrid materials using titanium phosphonate cluster. Polym J 49, 665–669 (2017). https://doi.org/10.1038/pj.2017.34
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pj.2017.34
This article is cited by
-
Preparation and properties of PDMS elastomer cross-linked with hydrolyzate of tetraethoxysilane, hexaethoxydisiloxane, and octaethoxytrisiloxane: influence of cross-linker structure
Journal of Sol-Gel Science and Technology (2024)
-
Hydrolysis and condensation behavior of tetraethoxysilane, hexaethoxydisiloxane, and octaethoxytrisiloxane
Journal of Sol-Gel Science and Technology (2023)
-
Characterization of NMR, IR, and Raman spectra for siloxanes and silsesquioxanes: a mini review
Journal of Sol-Gel Science and Technology (2022)
-
A review of phosphorus(V)-substituted titanium-oxo clusters
Journal of Sol-Gel Science and Technology (2021)
-
Preparation and properties of methyl- and cyclohexylsilsesquioxane oligomers as organic–inorganic fillers
Journal of Sol-Gel Science and Technology (2020)