Abstract
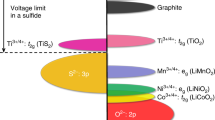
Lithium batteries have the highest energy density of all rechargeable batteries and are favoured in applications where low weight or small volume are desired — for example, laptop computers, cellular telephones and electric vehicles1. One of the limitations of present commercial lithium batteries is the high cost of the LiCoO2 cathode material. Searches for a replacement material that, like LiCoO2, intercalates lithium ions reversibly have covered most of the known lithium/transition-metal oxides, but the number of possible mixtures of these2,3,4,5 is almost limitless, making an empirical search labourious and expensive. Here we show that first-principles calculations can instead direct the search for possible cathode materials. Through such calculations we identify a large class of new candidate materials in which non-transition metals are substituted for transition metals. The replacement with non-transition metals is driven by the realization that oxygen, rather than transition-metal ions, function as the electron acceptor upon insertion of Li. For one such material, Li(Co,Al)O2, we predict and verify experimentally that aluminium substitution raises the cell voltage while decreasing both the density of the material and its cost.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Pistoia, G. Lithium Batteries (Elsevier, Amsterdam, 1994).
Delmas, C. & Saadoune, I. Electrochemical and physical properties of the LixNi1−yCoyO2phases. Solid State Ionics 53–56, 370–375 (1992).
Fey, G., Li, W. & Dahn, J. R. LiNiVO4: A 4.8 Volt electrode material for lithium cells. J. Electrochem. Soc. 141, 2279–2282 (1994).
Reimers, J. N., Rosen, E., Jones, C. D. & Dahn, J. R. Structure and electrochemistry of LixFeyNi1−y. Solid State Ionics 61, 335–334 (1993).
Ohzuku, T., Ueda, A., Nagayama, M., Iwakoshi, Y. & Komori, H. Comparitive study of LiCoO2, LiNi1/2Co1/2O2and LiNiO2 for 4 volt secondary lithium cells. Electrochim. Acta 38, 1159–1167 (1993).
Tarascon, J. M. & Guyomard, D. New electrolyte compositions stable over the 0 to 5 V voltage range and compatible with the Li1+xMn2O4/carbon Li-ion cells. Solid State Ionics 69, 293–305 (1994).
Aydinol, M. K., Kohan, A. F., Ceder, G., Cho, K. & Joannopoulos, J. Ab-initio study of lithium-intercalation in metal-oxides and metal-dichalcogenides. Phys. Rev. B 56, 1354–1365 (1997).
Ceder, G., Aydinol, M. K. & Kohan, A. F. Application of first-principles calculations to the design of rechargeable Li-batteries. Comput. Mater. Sci. 8, 161–169 (1996).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Nazri, G. A., Rougier, A. & Kia, K. F. in Solid State Chemistry of Inorganic Materials (eds Davies, P. K., Jacobson, A. J., Torardi, C. C. and Vanderah, T. A.) 635–646 (Mater. Res. Soc. Symp. Proc. 453, Materials Research Society, Pittsburgh, 1997).
Ohzuku, T., Ueda, A. & Kouguchi, M. Synthesis and characterization of LiAl1/4Ni3/4O2(R3m) for lithium-ion (shuttlecock) batteries. J. Electrochem. Soc. 142, 4033–4039 (1995).
Chiang, Y.-M. et al. Synthesis of LiCoO2 by decomposition and intercalation of hydroxides. J. Electrochem. Soc. 145, 887–891 (1998).
Acknowledgements
We thank A. Mayes for discussions and comments. This work was sponsored by Furukawa Electric and the US Department of Energy through the Idaho National Engineering Laboratory University Research Consortium.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ceder, G., Chiang, YM., Sadoway, D. et al. Identification of cathode materials for lithium batteries guided by first-principles calculations. Nature 392, 694–696 (1998). https://doi.org/10.1038/33647
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/33647
This article is cited by
-
Advances in 3D silicon-based lithium-ion microbatteries
Communications Materials (2024)
-
Learning heterogeneous reaction kinetics from X-ray videos pixel by pixel
Nature (2023)
-
A deep learning framework to emulate density functional theory
npj Computational Materials (2023)
-
Stable cycling of practical high-voltage LiCoO2 pouch cell via electrolyte modification
Nano Research (2023)
-
Efficient Direct Regeneration of Spent LiCoO2 Cathode Materials by Oxidative Hydrothermal Solution
JOM (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.