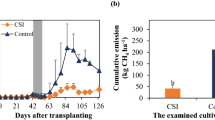
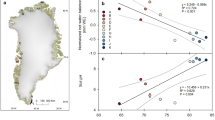
Abstract
Methane is involved in a number of chemical and physical processes in the Earth's atmosphere, including global warming1. Atmospheric methane originates mainly from biogenic sources, such as rice paddies and natural wetlands; the former account for at least 30% of the global annual emission of methane to the atmosphere2. As an increase of rice production by 60% is the most appropriate way to sustain the estimated increase of the human population during the next three decades3, intensified global fertilizer application will be necessary3: but it is known that an increase of the commonly used ammonium-based fertilizers can enhance methane emission from rice agriculture. Approximately 10–30% of the methane produced by methanogens in rice paddies is consumed by methane-oxidizing bacteria associated with the roots of rice4,5; these bacteria are generally thought to be inhibited by ammonium-based fertilizers, as was demonstrated for soils6,7,8 and sediments9,10. In contrast, we show here that the activity and growth of such bacteria in the root zone of rice plants are stimulated after fertilization. Using a combination of radioactive fingerprinting11 and molecular biology12 techniques, we identify the bacteria responsible for this effect. We expect that our results will make necessary a re-evaluation of the link between fertilizer use and methane emissions, with effects on global warming studies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Crutzen, P. J. in Ruminant Physiology: Digestion, Metabolism, Growth and Reproduction (eds von Engelhardt, W., Leonhardt-Marek, S., Breves, G. & Giesecke, D.) 291–315 (Enke, Stuttgart, Germany).
Neue, H. U. Fluxes of methane from rice fields and potential for mitigation. Soil Use Mgmt 13, 258–267 (1997).
Cassman, K. G. et al. Opportunities for increased nitrogen-use efficiency from improved resource management in irrigated rice systems. Field Crops Res. 56, 7–39 (1998 ).
Denier van der Gon, H. A. C. & Neue, H. U. Oxidation of methane in the rhizosphere of rice plants. Biol. Fertil. Soils 22, 359–366 (1996).
Bosse, U. & Frenzel, P. Activity and distribution of methane-oxidizing bacteria in flooded rice soil microcosms and in rice plants (Oryza sativa ). Appl. Environ. Microbiol. 63, 1199 –1207 (1997).
Steudler, P. A., Bowden, R. D., Mellilo, J. M. & Aber J. D. Influence of nitrogen fertilization on methane uptake in temperate forest soil. Nature 341, 314–316 ( 1989).
King, G. M. & Schnell, S. Effect of increasing atmospheric methane concentration on ammonium inhibition of soil methane consumption. Nature 370, 282–284 (1994).
Gulledge, J., Doyle, A. P. & Schimel, J. P. Different NH+4-inhibition patterns of soil CH4 –oxidizer populations across sites. Soil Biol. Biochem. 29, 13– 21 (1997).
Bosse, U., Frenzel, P. & Conrad, R. Inhibition of methane oxidation by ammonium in the surface layer of a littoral sediment. FEMS Microbiol. Ecol. 13, 123–134 (1993).
Van der Nat, F. J. W. A., DeBrouwer,, J. F. C., Middelburg, J. J. & Laanbroek, H. J. Spatial distribution and inhibition by ammonium of methane oxidation in intertidal freshwater marshes. Appl. Environ. Microbiol. 63, 4734–4740 (1997).
Roslev, P., Iversen, N. & Henriksen, K. Direct fingerprinting of metabolically active bacteria in environmental samples by substrate specific radiolabelling and lipid analysis. J. Microbiol. Methods 31, 99– 111 (1998).
Henckel, T., Friedrich, M. & Conrad, R. Molecular analysis of the methane-oxidizing microbial community in rice field soil by targeting the genes of the 16S rRNA, particulate methane monooxygenase, and methanol dehydrogenase. Appl. Environ. Microbiol. 65, 1980–1990 (1999).
Hanson, R. S. & Hanson, T. E. Methanotrophic bacteria. Microbiol. Rev. 60, 439–471 (1996).
King, G. M. Associations of methanotrophs with the roots and rhizomes of aquatic vegetation. Appl. Environ. Microbiol. 60, 3220– 3227 (1994).
Gilbert, B. & Frenzel, P. Rice roots and CH4 oxidation: the activity of bacteria, their distribution and the microenvironment. Soil Biol. Biochem. 30, 1903–1916.
Sundh, I., Borgå, P., Nilsson, M. & Svensson,, B. H. Estimation of cell numbers of methanotrophic bacteria in boreal peatlands based on analysis of specific phopholipid fatty acids. FEMS Microbiol. Ecol. 18, 103–112 ( 1995).
Bodelier, P. L. E., Hahn, A. P., Arth,, I. R & Frenzel,, P. Effects of ammonium-based fertilisation on microbial processes involved in methane emission from soils planted with rice. Biogeochemistry (submitted).
Dunfield, P. F. & Knowles, R. Kinetics of methane oxidation by nitrate, nitrite, and ammonium in a humisol. Appl. Environ. Microbiol. 61, 3129–3135 (1995).
King, G. M. & Schnell, S. Effects of ammonium and non-ammonium salt additions on methane oxidation by Methylosinus trichosporium OB3b and maine forest soil. Appl. Environ. Microbiol. 64 , 253–257 (1998).
Gulledge, J. & Schimel J. P. Low-concentration kinetics of atmospheric CH4 oxidation in soil and mechanism of NH+ 4 inhibition. Appl. Environ. Microbiol. 64, 4291–4298 (1998).
Lindau, C. W., Bollich, P. K., Delaune R. D., Patrick, W. H. Jr & Law, V. J. Effects of urea fertilizer and environmental factors on CH4 emissions from a Louisiana, USA rice field. Plant Soil 136, 195–203 (1991).
Banik, A., Sen, M. & Sen, S. P. Effects of inorganic fertilizers and micronutrients on methane production from wetland rice (Oryza sativa L.). Biol. Fertil. Soil 21, 319–322 ( 1996).
Bodelier, P. L. E., Wijlhuizen, A. G., Blom, C. W. P. M. & Laanbroek, H. J. Effects of photoperiod on growth of and denitrification by Pseudomonas chlororaphis in the root zone of Glyceria maxima, studied in a gnotobiotic microcosm. Plant Soil 190, 91 –103 (1997).
Bodelier, P. L. E. & Frenzel, P. Contribution of methanotrophic and nitrifying bacteria to CH4 and NH+ 4 oxidation in the rhizosphere of rice plants as determined by new methods of discrimination. Appl. Environ. Microbiol. 65, 1826–1833 (1999).
Strunk, O. & Ludwig,, W. ARB: A Software Environment for Sequence Data (Technische Universität München, Munich, Germany, 1996); available at http://www.biol.chemie.tu-muenchen.de/pub/ARB.
Ludwig, W. et al. Bacterial phylogeny based on comparative sequence analysis. Electrophoresis 19, 554–568 (1998).
Roslev, P. & Iversen, N. Radioactive fingerprinting of microorganisms that oxidize atmospheric methane in different soils. Appl. Environ. Microbiol. 65, 4064–4070 (1999).
Acknowledgements
We thank R. Conrad and P. Dunfield for comments on the manuscript, M. Friedrich for supporting the molecular studies, and B. Wagner and S. Fleisner for technical assistance. The project was supported by the EU, the Danish Technical Research Council and the Deutsche Forschungsgemeinschaft.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bodelier, P., Roslev, P., Henckel, T. et al. Stimulation by ammonium-based fertilizers of methane oxidation in soil around rice roots. Nature 403, 421–424 (2000). https://doi.org/10.1038/35000193
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35000193
This article is cited by
-
Temporal and environmental factors drive community structure and function of methanotrophs in volcanic forest soils
Journal of Forestry Research (2024)
-
Greenhouse gas emissions and mitigation in rice agriculture
Nature Reviews Earth & Environment (2023)
-
Soil nitrous oxide and methane fluxes from a land-use change transition of primary forest to oil palm in an Indonesian peatland
Biogeochemistry (2023)
-
Benefits and limitations of biochar for climate-smart agriculture: a review and case study from China
Biochar (2023)
-
Responses of anaerobic ammonia-oxidizing bacteria and methane-oxidizing archaea communities from different tillage modes in paddy fields
Journal of Soils and Sediments (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.