Abstract
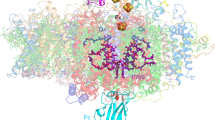
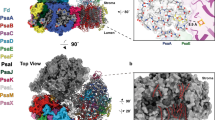
Oxygenic photosynthesis is the principal energy converter on earth. It is driven by photosystems I and II, two large protein–cofactor complexes located in the thylakoid membrane and acting in series. In photosystem II, water is oxidized; this event provides the overall process with the necessary electrons and protons, and the atmosphere with oxygen. To date, structural information on the architecture of the complex has been provided by electron microscopy of intact, active photosystem II at 15–30 Å resolution1, and by electron crystallography on two-dimensional crystals of D1-D2-CP47 photosystem II fragments without water oxidizing activity at 8 Å resolution2. Here we describe the X-ray structure of photosystem II on the basis of crystals fully active in water oxidation3. The structure shows how protein subunits and cofactors are spatially organized. The larger subunits are assigned and the locations and orientations of the cofactors are defined. We also provide new information on the position, size and shape of the manganese cluster, which catalyzes water oxidation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Nield, J. et al. Three-dimensional structure of Chlamydomonas reinhardtii and Synechococcus elongatus photosystem II complexes allow for comparison of their oxygen-evolving complex organisation. J. Biol. Chem. 275, 27940–27946 (2000).
Rhee, K.-H., Morris, E. P., Barber, J. & Kühlbrandt, W. Three-dimensional structure of photosystem II reaction centre at 8 Å resolution. Nature 396, 283–286 (1998).
Zouni, A., Jordan, R., Schlodder, E., Fromme, P. & Witt, H. T. First photosystem II crystals capable of water oxidation. Biochim. Biophys. Acta 1457, 103–105 (2000).
Witt, H. T. Primary reactions of oxygenic photosynthesis. Ber. BunsenGes. Phys. Chem. 100, 1923–1942 (1996).
Zouni, A. et al. in Photosynthesis: Mechanisms and Effects (ed. Garab, G.) 925–928 (Kluwer Academic, Dordrecht, 1998).
Barry, B. A., Boerner, R. J. & de Paula, J. C. in The Molecular Biology of Cyanobacteria (ed. Bryant, D. A.) 217–257 (Kluwer Academic, Dordrecht, 1994).
Svensson, B. et al. A model for the photosystem II reaction center core including the structure of the primary donor P680. Biochemistry 35, 14486–14502 (1996).
Xiong, J., Subramaniam, S. & Govindjee. A knowledge-based three dimensional model of the photosystem II reaction center of Chlamydomonas reinhardtii. Photosynth. Res. 56, 229–254 (1998).
Michel, H. & Deisenhofer, J. Relevance of the photosynthetic reaction center from purple bacteria to the structure of photosystem II. Biochemistry 27, 1–7 (1988).
Schubert, W.-D. et al. A common ancestor for oxygenic and anoxygenic photosynthetic systems: a comparison based on the structural model of photosystem I. J. Mol. Biol. 280, 297–314 (1998).
Harrer, R., Bassi, R., Testi, M. G. & Schäfer, C. Nearest-neighbor analysis of a photosystem II complex from Marchantia polymorpha L. (liverwort), which contains reaction center and antenna proteins. Eur. J. Biochem. 255, 196–205 (1998).
Ishikawa, Y. et al. Turnover of the aggregates and cross-linked products of the D1 protein generated by acceptor-side photoinhibition of photosystem II. Biochim. Biophys. Acta 1413, 147–158 (1999).
Rhee, K.-H. Three-dimensional Structure of Photosystem II Reaction Center by Electron Cryo-microscopy. Thesis, Univ. Heidelberg (1998).
Tomo, T., Enami, I. & Satoh, K. Orientation and nearest neighbor analysis of psbI gene product in the photosystem II reaction center complex using bifunctional cross-linkers. FEBS Lett. 323, 15–18 (1993).
Shi, L. X., Kim, S. J., Marchant, A., Robinson, C. & Schröder, W. P. Characterisation of the PsbX protein from photosystem II and light regulation of its gene expression in higher plants. Plant. Mol. Biol. 40, 737–744 (1999).
Summer, E. J., Schmid, V. H., Bruns, B. U. & Schmidt, G. W. Requirement for the H phosphoprotein in photosystem II of Chlamydomonas reinhardtii. Plant. Physiol. 113, 1359–1368 (1997).
Zheleva, D., Sharma, J., Panico, M., Morris, H. R. & Barber, J. Isolation and characterization of monomeric and dimeric CP47-reaction center photosystem II complexes. J. Biol. Chem. 273, 16122–16127 (1998).
Mayers, S. R. et al. Further characterization of the psbH locus of Synechocystis sp. PCC 6803: inactivation of psbH impairs QA to QB electron transport in photosystem 2. Biochemistry 32, 1454–1465 (1993).
Ahmed, A., Tajmir-Riahi, H. A. & Carpentier, R. A quantitative secondary structure analysis of the 33 kDa extrinsic polypeptide of photosystem II by FTIR spectroscopy. FEBS Lett. 363, 65–68 (1995).
Ghanotakis, D. F., Tsiotis, G. & Bricker T. M. in Concepts in Photobiology: Photosynthesis and Photomorphogenesis (eds Singhal, G. S., Renger, G., Sopory, S. K., Irrgang, K.-D. & Govindjee) 264–291 (Narosa, New Delhi, 1999).
Zech, S. G. et al. Pulsed EPR measurement of the distance between P680•- and QA•- in photosystem II. FEBS Lett. 414, 454–456 (1997).
Schelvis, J. P. M., van Noort, P. I., Aartsma, T. J. & van Gorkom, H. J. Energy transfer, charge separation and pigment arrangement in the reaction center of Photosystem II. Biochim. Biophys. Acta 1184, 242–250 (1994).
Ruffle, S., Hutchison, R. & Sayre, R. T. in Photosynthesis: Mechanisms and Effects (ed. Garab, G.) 1013–1016 (Kluwer Academic, Dordrecht, 1998).
Buser, C. A., Diner, B. A. & Brudvig, G. W. Photooxidation of cytochrome b559 in oxygen-evolving photosystem II. Biochemistry 31, 11449–11459 (1992).
Yachandra, V. K., Sauer, K. & Klein, M. P. Manganese cluster in photosynthesis: where plants oxidize water to dioxygen. Chem. Rev. 96, 2927–2950 (1996).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1996).
CCP4 Collaborative computational project number 4. The CCP4 suite: programmes for protein crystallography. Acta Cryst. D 50, 760–763 (1994).
Jones, T. A., Zou, J. Y., Cowan, S. W. & Kjeldgaard, M. Improved methods for binding protein models in electron density maps and the location of errors in these models. Acta Cryst. A 47, 110–119 (1991).
Esnouf, R. M. An extensively modified version of MolScript that includes greatly enhanced coloring capabilities. J. Mol. Graphics 15, 132–134 (1997).
Merritt, E. A. & Bacon, D. J. Raster3D—photorealistic molecular graphics. Methods Enzymol. 277, 505–524 (1997).
Acknowledgements
We thank C. Lüneberg, H. Schmidt and D. DiFiore for technical assistance, and W.-D. Schubert, R. Bittl, E. Schlodder, K. Irrgang and P. Jordan for discussions. Beamline assistance at DESY (Hamburg), ESRF (Grenoble) and Elettra (Trieste), and help of M. Burghammer with data collection at the manganese edge is gratefully acknowledged. We thank Egbert J. Boekema for providing us with electron micrographs of PSII. This work was supported by Deutsche Forschungsgemeinschaft, Sonderforschungsbereiche 312 and 498, BMBF (W.S.), and Fonds der Chemischen Industrie (W.S., H.-T.W., N.K. and P.F.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zouni, A., Witt, HT., Kern, J. et al. Crystal structure of photosystem II from Synechococcus elongatus at 3.8 Å resolution. Nature 409, 739–743 (2001). https://doi.org/10.1038/35055589
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35055589
This article is cited by
-
Importance of Manganese-Based Advanced Nanomaterial for Foliar Application
Journal of Cluster Science (2023)
-
Solar energy conversion by photosystem II: principles and structures
Photosynthesis Research (2023)
-
Theoretical elucidation of the structure, bonding, and reactivity of the CaMn4Ox clusters in the whole Kok cycle for water oxidation embedded in the oxygen evolving center of photosystem II. New molecular and quantum insights into the mechanism of the O–O bond formation
Photosynthesis Research (2023)
-
Photosystem 2 and the oxygen evolving complex: a brief overview
Photosynthesis Research (2022)
-
D‐Band EPR and ENDOR Spectroscopy of 15N‐Labeled Photosystem I
Applied Magnetic Resonance (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.