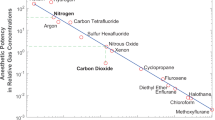
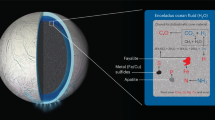
Abstract
Each recent report of liquid water existing elsewhere in the Solar System has reverberated through the international press and excited the imagination of humankind. Why? Because in the past few decades we have come to realize that where there is liquid water on Earth, virtually no matter what the physical conditions, there is life. What we previously thought of as insurmountable physical and chemical barriers to life, we now see as yet another niche harbouring 'extremophiles'. This realization, coupled with new data on the survival of microbes in the space environment and modelling of the potential for transfer of life between celestial bodies, suggests that life could be more common than previously thought. Here we examine critically what it means to be an extremophile, and the implications of this for evolution, biotechnology and especially the search for life in the Universe.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Macelroy, R. D. Some comments on the evolution of extremophiles. Biosystems 6, 74–75 (1974).
Madigan, M. T. & Marrs, B. L. Extremophiles. Sci. Am. 276, 82–87 (1997).
Horikoshi, K. & Grant, W. D. Extremophiles. Microbial Life in Extreme Environments (Wiley-Liss, New York, 1998).
Seckbach, J. (ed.) Journey to Diverse Microbial Worlds: Adaptation to Exotic Environments (Kluwer, Dordrecht, 2000).
Cowan, D. Hot bugs, cold bugs and sushi. Trends Biotechnol. 16, 241–242 (1998).
Aguilar, A., Ingemansson, T. & Magnien, E. Extremophile microorganisms as cell factories: support from the European Union. Extremophiles 2, 367–373 (1998).
Tyrell, R. M. in Oxidative Stress: Oxidants and Antioxidants (ed. Sies, H.) 57–83 (Academic, London, 1991).
Newcomb, T. G. & Loeb, L. A. in DNA Damage and Repair, Vol. 1: DNA Repair in Prokaryotes and Lower Eukaryotes (eds Nickoloff, J. A. & Hoekstra, M. F.) 65–84 (Humana, Totowa, NJ, 1998).
Minton, K. W. DNA repair in the extremely radioresistant bacterium Deinococcus radiodurans. Mol. Microbiol. 13, 9–15 (1994).
Chow, F. I. & Tan, S. T. Manganese(II) induces cell division and increases in superoxide dismutase and catalase activities in an aging deinococcal culture. J. Bacteriol. 172, 2029–2035 (1990).
Venkateswaran, A. et al. Physiologic determinants of radiation resistance in Deinococcus radiodurans. Appl. Environ. Microbiol. 66, 2620–2626 (2000).
Seki, K. & Toyoshima, M. Preserving tardigrades under pressure. Nature 395, 853–854 (1998).
Ball, P. H2O. A Biography of Water (Weldenfeld & Nicolson, London, 1999).
Morita, R. Y. Psychrophilic bacteria. Bacteriol. Rev. 39, 144–167 (1975).
Blochl, E. et al. Pyrolobus fumarii, gen. and sp. nov., represents a novel group of archaea, extending the upper temperature limit for life to 113 °C. Extremophiles 1, 14–21 (1997).
Schuliger, J. W., Brown, S. H., Baross, J. A. & Kelly, R. M. Purification and characterization of a novel amylolytic enzyme from ES4, a marine hyperthermophilic archaeum. Mol. Mar. Biol. Biotech. 2, 76–87 (1993).
Clarke, A. in Evolution on Planet Earth: The Impact of the Physical Environment (eds Rothschild, L. & Lister, A.) (Academic, London, in the press).
Kohshima, S. A novel cold-tolerant insect found in a Himalayan glacier. Nature 310, 225 (1984).
Franks, F. Biophysics and Biochemistry at Low Temperatures (Cambridge Univ. Press, Cambridge, 1985).
Wharton, D. A. & Ferns, D. J. Survival of intracellular freezing by the Antarctic nematode Panagrolaimus davidi. J. Exp. Biol. 198, 1381–1387 (1995).
Battista, J. R. Against all odds: the survival strategies of Deinococcus radiodurans. Annu. Rev. Microbiol. 51, 203–224 (1997).
Ferreira, A. C. et al. Characterization and radiation resistance of new isolates of Rubrobacter radiotolerans and Rubrobacter xylanophilus. Extremophiles 3, 235–238 (1999).
Ben-Amotz, A. & Avron, M. Dunaliella bardawil can survive especially high irradiance levels by the accumulation of β-carotene. Trends Biotechnol. 8, 121–126 (1990).
Pledger, R. J., Crump, B. C. & Baross, J. A. A barophilic response by two hyperthermophilic, hydrothermal vent Archaea: an upward shift in the optimal temperature and acceleration of growth rate at supra-optimal temperatures by elevated pressure. FEMS Microbiol. Ecol. 14, 233–242 (1994).
Bartlett, D. H. & Bidle, K. A. in Enigmatic Microorganisms and Life in Extreme Environments (ed. Seckbach, J.) 503–512 (Kluwer, Dordrecht, 1999).
Van Dover, C. L. The Ecology of Deep-Sea Hydrothermal Vents (Princeton Univ. Press, Princeton, 2000).
Kato, C. et al. Extremely barophilic bacteria isolated from the Mariana Trench, Challenger Deep, at a depth of 11,000 meters. Appl. Environ. Microbiol. 64, 1510–1513 (1998).
Cogoli, A., Iversen, T. H., Johnsson, A., Mesland, D. & Oser, H. European Space Agency Spec. Publ. No. 1105, 49–64 (1989)
Crowe, J. H. Anhydrobiosis: an unsolved problem. Am. Nat. 105, 563–574 (1971).
Wright, J. C. Desiccation tolerance and water-retentive mechanisms in tardigrades. J. Exp. Biol. 142, 267–292 (1989).
Glasheen, J. S. & Hand, S. C. Anhydrobiosis in embryos of the brine shrimp Artemia: characterization of metabolic arrest during reductions in cell-associated water. J. Exp. Biol. 135, 363–389 (1988).
Potts, M. Desiccation tolerance of prokaryotes. Microbiol. Rev. 58, 755–805 (1994).
Cox, C. S. Roles of water molecules in bacteria and viruses. Origins Life 23, 29–36 (1993).
Dose, K. et al. ERA-experiment: space biochemistry. Adv. Space Res. 16(8), 119–129 (1995).
Dose, K. & Gill, M. DNA stability and survival of Bacillus subtilis spores in extreme dryness. Origins Life 25, 277–293 (1994).
Seckbach, J. in Enigmatic Microorganisms and Life in Extreme Environments (ed. Seckbach, J.) 427–435 (Kluwer, Dordrecht, 1999).
Doemel, W. N. & Brock, T. D. The physiological ecology of Cyanidium caldarium. J. Gen. Microbiol. 67, 17–32 (1971).
Pick, U. in Enigmatic Microorganisms and Life in Extreme Environments (ed. Seckbach, J.) 467–478 (Kluwer, Dordrecht, 1999).
Schleper, C., Pühler, G., Kühlmorgen, B. & Zillig, W. Life at extremely low pH. Nature 375, 741–742 (1995).
Schleper C. et al. Picrophilus gen. nov., fam. nov.: a novel aerobic, heterotrophic, thermoacidophilic genus and family comprising archaea capable of growth around pH 0. J. Bacteriol. 177, 7050–7059 (1995).
Edwards, K. J., Bond, P. L., Gihring, T. M. & Banfield, J. F. An archaeal iron-oxidizing extreme acidophile important in acid mine drainage. Science 287, 1796–1799 (2000).
Krulwich, T. A., Ito, M., Hicks, D. B., Gilmour, R. & Guffanti, A. A. pH homeostasis and ATP synthesis: studies of two processes that necessitate inward proton translocation in extremely alkaliphilic Bacillus species. Extremophiles 2, 217–222 (1998).
Javor, B. Hypersaline Environments (Springer, Berlin, 1989).
Jones, B. E., Grant, W. D., Duckworth, A. W. & Owenson, G. G. Microbial diversity of soda lakes. Extremophiles 2, 191–200 (1998).
Beckman, K. B. & Ames, B. N. The free radical theory of aging matures. Physiol. Rev. 78, 547–581 (1998).
Pourzand, C. & Tyrrell, R. M. Apoptosis, the role of oxidative stress and the example of solar UV radiation. Photochem. Photobiol. 70, 380–390 (1999).
Cooper, W. & Lean, D. in Encyclopedia of Earth System Science Vol. 2 (ed. Nierenber, W. A.) 527–535 (Academic, San Diego, 1992).
Seckbach, J., Baker, F. A. & Shugarman, P. M. Algae survive under pure CO2 . Nature 227, 744–745 (1970).
Nies, D. H. Heavy metal-resistant bacteria as extremophiles: molecular physiology and biotechnological use of Ralstonia sp. CH34. Extremophiles 4, 77–82 (2000).
Isken, S. & de Bont, J. A. M. Bacteria tolerant to organic solvents. Extremophiles 2, 229–238 (1998).
Beardall, J. & Entwisle, L. Internal pH of the obligate acidophile Cyanidium caldarium Geitler (Rhodophyta?). Phycologia 23, 397–399 (1984).
Jaenicke, R. Stability and folding of ultrastable proteins: eye lens crystallins and enzymes from thermophiles. FASEB J. 10, 84–92 (1996).
Peak, M. J., Robb, F. T. & Peak, J. G. Extreme resistance to thermally induced DNA backbone breaks in the hyperthermophilic archaeon Pyrococcus furiosus. J. Bacteriol. 177, 6316–6318 (1995).
Marguet, E. & Forterre, P. Protection of DNA by salts against thermodegradation at temperatures typical for hyperthermophiles. Extremophiles 2, 115–122 (1998).
Galtier, N., Tourasse, N. & Gouy, M. A nonhyperthermophilic common ancestor to extant life forms. Science 283, 220–221 (1999).
Galtier, N. & Lobry, J. R. Relationships between genomic G+C content, secondary structures and optimal growth temperature in prokaryotes. J. Mol. Evol. 44, 632 (1997).
Storey, K. B. & Storey, J. M. Natural freezing survival in animals. Annu. Rev. Ecol. Syst. 27, 365–386 (1996).
Russell, N. J. Toward a molecular understanding of cold activity of enzymes from psychrophiles. Extremophiles 4, 83–90 (2000).
Cummings, S. P. & Black, G.W. Polymer hydrolysis in a cold climate. Extremophiles 3, 81–87 (1999).
Aghajari, N., Feller, G., Gerday, C. & Haser, R. Structures of the psychrophilic Alteromonas haloplanctis α-amylase give insights into cold adaptation at a molecular level. Structure 6, 1503–1516 (1998).
Willem, S. et al. Protein adaptation to low temperatures: a comparative study of α-tubulin sequences in mesophilic and psychrophilic algae. Extremophiles 3, 221–226 (1999).
Rothschild, L. J. in Enigmatic Microorganisms and Life in Extreme Environments (ed. Seckbach, J.) 551–562 (Kluwer, Dordrecht, 1999).
Battista, J. R. in DNA Damage and Repair, Vol. I: DNA Repair in Prokaryotes and Lower Eukaryotes (eds Nickoloff, J. A. & Hoekstra, M. F.) 287–303 (Humana, Totowa, NJ, 1998).
Nakasone, K., Ikegami, A., Kato, C., Usami, R. & Horikoshi, K. Mechanisms of gene expression controlled by pressure in deep-sea microorganisms. Extremophiles 2, 149–154 (1998).
Abe, F., Kato, C. & Horikoshi, K. Pressure-regulated metabolism in microorganisms. Trends Microbiol. 7, 447–453 (1999).
Yancey, P. H., Clark, M. E., Hand, S. C., Bowlus, R. D. & Somero, G. N. Living with water stress: evolution of osmolyte systems. Science 217, 1214–1216 (1982).
Larsen, H. Biochemical aspects of extreme halophilism. Adv. Microb. Physiol. 1, 97–132 (1967).
Le Rudulier, D. & Bouillard, L. Glycine Betaine, an osmotic effector in Klebsiella pneumonia and other members of the Enterobacteriaceae. Appl. Environ. Microbiol. 46, 152–159 (1983).
Crowe, J. H., Hoekstra, F. A. & Crowe, L. M. Anhydrobiosis. Annu Rev. Physiol. 54, 579–599 (1992).
Wehner, J. & Horneck, G. Effects of vacuum UV and UVC radiation on dry E. coli plasmid pUC19 II. Mutational specificity at the lacZ gene. J. Photochem. Photobiol. B 30, 171–177 (1995).
Wehner, J. & Horneck, G. Effects of vacuum UV and UVC radiation on dry E. coli plasmid pUC19 I. Inactivation, lacZ− mutation induction and strand breaks. J. Photochem. Photobiol. B 28, 77–85 (1995).
Brock, T. D. Thermophilic Microorganisms and Life at High Temperatures (Springer, New York, 1978).
Reysenbach, A. L., Voytek, M. & Mancinelli, R. L. (eds) Microbiology of Yellowstone (Kluwer, New York, in the press).
Horikoshi, K. Barophiles: deep-sea microorganisms adapted to an extreme environment. Curr. Opin. Microbiol. 1, 291–295 (1998).
Kennish, M. J. (ed.) Practical Handbook of Marine Science 2nd edn 236–237 (CRC Press, Boca Raton, 1994).
Karl, D. M. (ed.) The Microbiology of Deep-sea Hydrothermal Vents (CRC Press, Boca Raton, 1995).
Cody, G. D. et al. Primordial carbonylated iron-sulfur compounds and the synthesis of pyruvate. Science 289, 1337–1340 (2000).
Pace, N. A molecular view of microbial diversity and the biosphere. Science 276, 734–740 (1997).
Sleep, N. H., Zahnle, K. J., Kasting, J. F. & Morowitz, H. J. Annihilation of ecosystems by large impacts on early earth. Nature 342, 139–142 (1989).
Oren, A. in Enigmatic Microorganisms and Life in Extreme Environments (ed. Seckbach, J.) 339–355 (Kluwer, Dordrecht, 1999).
Kamekura, M. Diversity of extremely halophilic bacteria. Extremophiles 2, 289–295 (1998).
Bell, C. M. Saline lake carbonates within an Upper Jurassic-Lower Cretaceous continental red bed sequence in the Atacama region of northern Chile. Sedimentology 36, 651–664 (1989).
Castanier, S., Perthuisot, J.-P., Rouchy, J.-M., Maurin, A. & Guelorget. O. Halite ooids in Lake Asal Djibouti biocrystalline build-ups. Geobios (Lyon) 25, 811–821 (1992).
Norton, C. F. & Grant, W. D. Survival of halobacteria within fluid inclusions in salt crystals. J. Gen. Microbiol. 134, 1365–1373 (1988).
Rothschild, L. J., Giver, L. J., White, M. R. & Mancinelli, R. L. Metabolic activity of microorganisms in gypsum-halite crusts. J. Phycol. 30, 431–438 (1994).
Vreeland, R. H., Rosenzweig, W. D. & Powers, D. W. Isolation of a 250 million-year-old halotolerant bacterium from a primary salt crystal. Nature 407, 897–900 (2000).
Evans, R. D. & Johansen, J. R. Microbiotic crusts and ecosystem processes. Crit. Rev. Plant Sci. 18, 182–225 (1999).
Rundel, P. W. et al. The phytogeography and ecology of the coastal Atacama and Peruvian deserts. ALISO 13, 1–49 (1991).
van Thielen, N. & Garbary, D. J. in Enigmatic Microorganisms and Life in Extreme Environments (ed. Seckbach, J.) 245–253 (Kluwer, Dordrecht, 1999).
Friedmann, E. I. Endolithic microorganisms in the Antarctic cold desert. Science 215, 1045–1053 (1982).
Bidigare, R. R. et al. Evidence for a photoprotective function for secondary carotenoids of snow algae. J. Phycol. 29, 427–434 (1993).
Junge, K., Krembs, C., Deming, J., Stierle, A. & Eicken, H. A microscopic approach to investigate bacteria under in-situ conditions in sea-ice samples. Ann. Glaciol. (in the press).
Friedmann, E. I. Viable Microorganisms in Permafrost (ed. Gilichinsky, D. A.) 21–26 (Institute of Soil Science and Photosynthesis, Russian Academy of Science, Pushchino, 1994); cited in Vishnivetskaya, T., Kathariou, S., McGrath, J., Gilichinsky, D. & Tiedje, J. M. Low-temperature recovery strategies for the isolation of bacteria from ancient permafrost sediments. Extremophiles 4, 165–173 (2000).
Staley, J. T. & Gosink, J. J. Poles apart: biodiversity and biogeography of sea ice bacteria. Annu. Rev. Microbiol. 53, 189–215 (1999).
Mancinelli, R. L. & Shulls, W. A. Airborne bacteria in an urban environment. Appl. Environ. Microbiol. 35, 1095–1101 (1978).
Cox, C. S. Roles of Maillard Reactions in Diseases (HMSO, London, 1991).
Israeli, E., Gitelman, J. & Lighthart, B. in Atmospheric Microbial Aerosol Theory and Application (eds Lighthart, B. & Mohr, A. J.) 166–192 (Chapman & Hall, New York, 1994).
Cox, C. S. The Aerobiological Pathway of Microorganisms (Wiley, New York, 1987).
Cox, C. S. & Wathes, C. M. Bioaerosols Handbook (Lewis, London, 1995).
Lighthart, B. & Mohr, A. J. (eds) Atmospheric Microbial Aerosol Theory and Application 68–98, 166–192 (Chapman & Hall, New York, 1994).
Marchant, J. Life from the skies—did droplets high in the atmosphere give birth to the first living cells? New Sci. 2247, 4–5 (15 July 2000).
Dundas, I. Was the environment for primordial life hypersaline? Extremophiles 2, 375–377 (1998).
Biemann, K. et al. The search for organic substances and inorganic volatile compounds in the surface of Mars. J. Geophys. Res. 82, 4641–4658 (1977).
Malin, M. C. & Edgett, K. S. Evidence for recent groundwater seepage and surface runoff on Mars. Science 288, 2330–2335 (2000).
Horneck, G. in Evolution on Planet Earth: The Impact of the Physical Environment (eds Rothschild, L. & Lister, A.) (Academic, London, in the press).
Rothschild, L. J. Earth analogs for Martian life. Microbes in evaporites, a new model system for life on Mars. Icarus 88, 246–260 (1990).
Mancinelli, R. L. & Klovstad, M. Survival of Bacillus subtilis spores on space craft surfaces. Planet. Space Sci. 48, 1093–1097 (2000).
Chyba, C. Energy for microbial life on Europa. Nature 403, 381–382 (2000).
Stone, R. Permafrost comes alive for Siberian researchers. Science 286, 36–37 (1999).
Richter, H. Zur Darwinschen Lehre. Schmidts Jahrb. Ges. Med. 126, 243–249 (1865).
Thomson, W. in Popular Lectures and Addresses 132–205 (Macmillan, New York, 1894).
Arrhenius, S. Die Verbreitung des Lebens im Weltenraum. Umschau 7, 481–485 (1903).
Nicholson, W. L., Munakata, N., Horneck, G., Melosh, H. J. & Setlow, P. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol. Mol. Biol Rev. 64, 548–572 (2000).
Rothschild, L. J. in Evolution on Planet Earth: The Impact of the Physical Environment (eds Rothschild, L. & Lister, A.) (Academic, London, in the press).
Horneck, G., Krasavin, E. A. & Kozubek, S. Mutagenic effects of heavy ions in bacteria. Adv. Space Res. 14(10), 315–329 (1994).
Cheng, C-H. C. & Chen, L. Evolution of an antifreeze glycoprotein. Nature 401, 463–464 (1999).
Ito, S. et al. Alkaline detergent enzymes from alkaliphiles: enzymatic properties, genetics, and structures. Extremophiles 2, 185–190 (1998).
Zeikus, J. G., Vielle, C. & Savchenko, A. Thermozymes: biotechnology and structure-function relationships. Extremophiles 2, 179–182 (1998).
Hough, D. W. & Danson, M. J. Extremozymes. Curr. Opin. Chem. Biol. 3, 39–46 (1999).
Sellek, G. A. & Chaudhuri, J. B. Biocatalysis in organic media using enzymes from extremophiles. Enzyme Microb. Technol. 25, 471–482 (1999).
Brock, T. D. & Freeze, H. Thermus aquaticus gen. n., a nonsporulating extreme thermophile. J. Bacteriol. 98, 289–297 (1969).
Mattila, P., Korpela, J., Tenkanen, T. & Pitkänen, K. Fidelity of DNA synthesis by the Thermococcus litoralis DNA polymerase—an extremely heat stable enzyme with proofreading activity. Nucleic Acids Res. 19, 4967–4973 (1991).
Cariello, N. F., Swenberg, J. A. & Skopek, T. R. Fidelity of Thermococcus litoralis DNA polymerase (Vent) in PCR determined by denaturing gradient gel electrophoresis. Nucleic Acids Res. 19, 4193–4198 (1991).
Nichols, D. et al. Developments with Antarctic microorganisms: culture collections, bioactivity screening, taxonomy, PUFA production and cold-adapted enzymes. Curr. Opin. Biotechnol. 10, 240–246 (1999).
Ben-Amotz, A. in Enigmatic Microorganisms and Life in Extreme Environments (ed. Seckbach, J.) 401–410 (Kluwer, Dordrecht. 1999).
Acknowledgements
We thank the many people who were generous with information, especially: J. Baross on hydrothermal vents; L. Giver and C. Wong on commercial aspects; G. Antranikian and M. Meyer on government programmes; J. Deming, K. Junge, P. Ball, S. Emerson and G. Packard on life at low temperatures; and K. Stedman for life at high temperatures. A. Deutch, K. Duffy and S. Sturtevant provided tips on the thermophiles of Yellowstone. E. Holton, D. Cowan and J. Parkes provided helpful reviews.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Rothschild, L., Mancinelli, R. Life in extreme environments. Nature 409, 1092–1101 (2001). https://doi.org/10.1038/35059215
Issue Date:
DOI: https://doi.org/10.1038/35059215
This article is cited by
-
Young volcanic terrains are windows into early microbial colonization
Communications Earth & Environment (2024)
-
Molecular Biology Applications of Psychrophilic Enzymes: Adaptations, Advantages, Expression, and Prospective
Applied Biochemistry and Biotechnology (2024)
-
Unveiling microbial diversity in deep geothermal fluids, from current knowledge and analogous environments
Geothermal Energy (2023)
-
Environment and taxonomy shape the genomic signature of prokaryotic extremophiles
Scientific Reports (2023)
-
Exploring microbial diversity in hot springs of Surajkund, India through 16S rRNA analysis and thermozyme characterization from endogenous isolates
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.