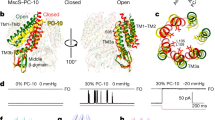
Abstract
Mechanosensitive channels act as membrane-embedded mechano-electrical switches, opening a large water-filled pore in response to lipid bilayer deformations. This process is critical to the response of living organisms to direct physical stimulation, such as in touch, hearing and osmoregulation. Here, we have determined the structural rearrangements that underlie these events in the large prokaryotic mechanosensitive channel (MscL) using electron paramagnetic resonance spectroscopy and site-directed spin labelling. MscL was trapped in both the open and in an intermediate closed state by modulating bilayer morphology. Transition to the intermediate state is characterized by small movements in the first transmembrane helix (TM1). Subsequent transitions to the open state are accompanied by massive rearrangements in both TM1 and TM2, as shown by large increases in probe dynamics, solvent accessibility and the elimination of all intersubunit spin–spin interactions. The open state is highly dynamic, supporting a water-filled pore of at least 25 Å, lined mostly by TM1. These structures suggest a plausible molecular mechanism of gating in mechanosensitive channels.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Sukharev, S. I., Blount, P., Martinac, B. & Kung, C. Mechanosensitive channels of Escherichia coli: the MscL gene, protein, and activities. Annu. Rev. Physiol. 59, 633–657 (1997)
Wood, J. M. Osmosensing by bacteria: signals and membrane-based sensors. Microbiol. Mol. Biol. Rev. 63, 230–262 (1999)
Hamill, O. P. & Martinac, B. Molecular basis of mechanotransduction in living cells. Physiol. Rev. 81, 685–740 (2001)
Martinac, B., Adler, J. & Kung, C. Mechanosensitive ion channels of E. coli activated by amphipaths. Nature 348, 261–263 (1990)
Sukharev, S. I., Blount, P., Martinac, B., Blattner, F. R. & Kung, C. A large-conductance mechanosensitive channel in E. coli encoded by mscL alone. Nature 368, 265–268 (1994)
Patel, A. J. et al. A mammalian two pore domain mechano-gated S-like K + channel. EMBO J. 17, 4283–4290 (1998)
Levina, N. et al. Protection of Escherichia coli cells against extreme turgor by activation of MscS and MscL mechanosensitive channels: identification of genes required for MscS ativity. EMBO J. 18, 1730–1737 (1999)
Chang, G., Spencer, R. H., Lee, A. T., Barclay, M. T. & Rees, D. C. Structure of the MscL homolog from Mycobacterium tuberculosis: a gated mechanosensitive ion channel. Science 282, 2220–2226 (1998)
Ou, X., Blount, P., Hoffman, R. J. & Kung, C. One face of a transmembrane helix is crucial in mechanosensitive channel gating. Proc. Natl Acad. Sci. USA 95, 11471–11475 (1998)
Yoshimura, K., Batiza, A., Schroeder, M., Blount, P. & Kung, C. Hydrophilicity of a single residue residue within MscL correlates with increased channel mechanosensitivity. Biophys. J. 77, 1960–1972 (1999)
Yoshimura, K., Batiza, A. & Kung, C. Chemically charging the pore constriction opens the mechanosensitive channel MscL. Biophys. J. 80, 2198–2206 (2001)
Cruickshank, C. C., Minchin, R. F., Le Dain, A. C. & Martinac, B. Estimation of the pore size of the large-conductance mechanosensitive ion channel of Escherichia coli. Biophys. J. 73, 1925–1931 (1997)
Spencer, R. H., Chang, G. & Rees, D. C. ‘Feeling the pressure’: structural insights into a gated mechanosensitive channel. Curr. Opin. Struct. Biol. 9, 448–454 (1999); erratum 9, 650–651 (1999)
Batiza, A. F., Rayment, I. & Kung, C. Channel gate: Tension, leak and disclosure. Struct. Fold. Design 7, R99–R103 (1999)
Sukharev, S., Betanzos, M., Chiang, C.-S. & Guy, H. The gating mechanism of the large mechanosensitive channel MscL. Nature 409, 720–724 (2001)
Sukharev, S., Durell, S. R. & Guy, H. R. Structural models of the MscL gating mechanism. Biophys. J. 81, 917–936 (2001)
Perozo, E., Kloda, A., Cortes, D. M. & Martinac, B. Physical principles underlying the transduction of bilayer deformation forces during mechanosensitive channel gating. Nature Struct. Biol. 9, 696–703 (2002).
Maingret, F., Patel, A. J., Lesage, F., Lazdunski, M. & Honoré, E. Lysophospholipids open the two-pore domain mechano-gated K+ channels TREK-1 and TRAAK. J. Biol. Chem. 275, 10128–10133 (2000)
Hubbell, W. L., Gross, A., Langen, R. & Lietzow, M. A. Recent advances in site-directed spin labeling of proteins. Curr. Opin. Struct. Biol. 8, 649–656 (1998)
Hubbell, W. L., Cafiso, D. S. & Altenbach, C. Identifying conformational changes with site-directed spin labeling. Nature Struct. Biol. 7, 735–739 (2000)
Mchaourab, H. & Perozo, E. in Biological Magnetic Resonance Vol. 19 (eds Eaton, G. R., Eaton, S. S. & Berliner, L. J.) 155–218 (Kluwer-Plenum, New York, 2000)
Perozo, E., Kloda, A., Cortes, D. M. & Martinac, B. Site-directed spin-labeling analysis of reconstituted Mscl in the closed state. J. Gen. Physiol. 118, 193–206 (2001)
Sompornpisut, P., Liu, Y. S. & Perozo, E. Calculation of rigid-body conformational changes using restraint-driven Cartesian transformations. Biophys. J. 81, 2530–2546 (2001)
Liu, Y. S., Sompornpisut, P. & Perozo, E. Structure of the KcsA channel intracellular gate in the open state. Nature Struct. Biol. 8, 883–887 (2001)
Lee, B. & Richards, F. M. The interpretation of protein structures: estimation of static accessibility. J. Mol. Biol. 55, 379–400 (1971)
Martinac, B., Buechner, M., Delcour, A. H., Adler, J. & Kung, C. Pressure-sensitive ion channel in Escherichia coli. Proc. Natl Acad. Sci. USA 84, 2297–2301 (1987)
Martinac, B. Mechanosensitive channels in prokaryotes. Cell Physiol. Biochem. 11, 61–76 (2001)
Gullingsrud, J., Kosztin, D. & Schulten, K. Structural determinants of MscL gating studied by molecular dynamics simulations. Biophys. J. 80, 2074–2081 (2001)
Smart, O. S., Neduvelil, J. G., Wang, X., Wallace, B. A. & Sansom, M. S. HOLE: a program for the analysis of the pore dimensions of ion channel structural models. J. Mol. Graph. 14, 354–360 (1996)
Sukharev, S. I., Sigurdson, W. J., Kung, C. & Sachs, F. Energetic and spatial parameters for gating of the bacterial large conductance mechanosensitive channel, MscL. J. Gen. Physiol. 113, 525–540 (1999)
Perozo, E., Cortes, D. M. & Cuello, L. G. Three-dimensional architecture and gating mechanism of a K+ channel studied by EPR spectroscopy. Nature Struct. Biol. 5, 459–469 (1998)
Cortes, D. M., Cuello, L. G. & Perozo, E. Molecular architecture of full-length KcsA: role of cytoplasmic domains in ion permeation and activation gating. J. Gen. Physiol. 117, 165–180 (2001)
Altenbach, C., Marti, T., Khorana, H. G. & Hubbell, W. L. Transmembrane protein structure: spin labeling of bacteriorhodopsin mutants. Science 248, 1088–1092 (1990)
Abragam, A. Principles of Nuclear Magnetism (Oxford Univ. Press, Oxford, 1961)
Sompornpisut, P., Mchaourab, H. & Perozo, E. Spectroscopically-determined solvent accessibilities as constraints for global fold discrimination in proteins. Biophys. J. 82, 474a (2002)
Case, D. A. et al. AMBER 6 (Univ. California, San Francisco, 1999)
Laskowski, R. A., Rullmannn, J. A., MacArthur, M. W., Kaptein, R. & Thornton, J. M. AQUA and PROCHECK-NMR: programs for checking the quality of protein structures solved by NMR. J. Biomol. NMR 8, 477–486 (1996)
Nicholls, A., Sharp, K. A. & Honig, B. Protein folding and association: insights from the interfacial and thermodynamic properties of hydrocarbons. Proteins 11, 281–296 (1991)
Acknowledgements
We thank R. Nakamoto and M. Wiener for thoughtful discussions, C. Ptak for reading the manuscript, and S. Mochel for encouragement. This work was supported in part by the NIH (E.P.) and the McKnight endowment fund for neuroscience (E.P.), the Australian Research Council grants (B.M.) and the Australian Academy of Science (Scientific Visit Award to B.M). Transmembrane segment coordinates in the three conformations have been deposited in the Protein Data Bank (accession codes 1KYK, 1KYL and 1KYM).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Rights and permissions
About this article
Cite this article
Perozo, E., Cortes, D., Sompornpisut, P. et al. Open channel structure of MscL and the gating mechanism of mechanosensitive channels. Nature 418, 942–948 (2002). https://doi.org/10.1038/nature00992
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature00992
This article is cited by
-
Mechanosensitive aquaporins
Biophysical Reviews (2023)
-
Asymmetric effects of amphipathic molecules on mechanosensitive channels
Scientific Reports (2022)
-
Building programmable multicompartment artificial cells incorporating remotely activated protein channels using microfluidics and acoustic levitation
Nature Communications (2022)
-
MCAs in Arabidopsis are Ca2+-permeable mechanosensitive channels inherently sensitive to membrane tension
Nature Communications (2021)
-
Role of adhesion forces in mechanosensitive channel gating in Staphylococcus aureus adhering to surfaces
npj Biofilms and Microbiomes (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.