Abstract
Targeted, temporally regulated neural modulation is invaluable in determining the physiological roles of specific neural populations or circuits. Here we describe a system for non-invasive, temporal activation or inhibition of neuronal activity in vivo and its use to study central nervous system control of glucose homeostasis and feeding in mice. We are able to induce neuronal activation remotely using radio waves or magnetic fields via Cre-dependent expression of a GFP-tagged ferritin fusion protein tethered to the cation-conducting transient receptor potential vanilloid 1 (TRPV1) by a camelid anti-GFP antibody (anti-GFP–TRPV1)1. Neuronal inhibition via the same stimuli is achieved by mutating the TRPV1 pore, rendering the channel chloride-permeable. These constructs were targeted to glucose-sensing neurons in the ventromedial hypothalamus in glucokinase–Cre mice, which express Cre in glucose-sensing neurons2. Acute activation of glucose-sensing neurons in this region increases plasma glucose and glucagon, lowers insulin levels and stimulates feeding, while inhibition reduces blood glucose, raises insulin levels and suppresses feeding. These results suggest that pancreatic hormones function as an effector mechanism of central nervous system circuits controlling blood glucose and behaviour. The method we employ obviates the need for permanent implants and could potentially be applied to study other neural processes or used to regulate other, even dispersed, cell types.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Stanley, S. A., Sauer, J., Kane, R. S., Dordick, J. S. & Friedman, J. M. Remote regulation of glucose homeostasis in mice using genetically encoded nanoparticles. Nature Med. 21, 92–98 (2015)
Stanley, S. et al. Profiling of glucose-sensing neurons reveals that GHRH neurons are activated by hypoglycemia. Cell Metab. 18, 596–607 (2013)
Davies, R., Nakajima, S. & White, N. Enhancement of feeding produced by stimulation of the ventromedial hypothalamus. J. Comp. Physiol. Psychol. 86, 414–419 (1974)
Goto, Y., Carpenter, R. G., Berelowitz, M. & Frohman, L. A. Effect of ventromedial hypothalamic lesions on the secretion of somatostatin, insulin, and glucagon by the perfused rat pancreas. Metabolism 29, 986–990 (1980)
Shimazu, T., Fukuda, A. & Ban, T. Reciprocal influences of the ventromedial and lateral hypothalamic nuclei on blood glucose level and liver glycogen content. Nature 210, 1178–1179 (1966)
Schwartz, M. W. et al. Cooperation between brain and islet in glucose homeostasis and diabetes. Nature 503, 59–66 (2013)
Kang, L., Routh, V. H., Kuzhikandathil, E. V., Gaspers, L. D. & Levin, B. E. Physiological and molecular characteristics of rat hypothalamic ventromedial nucleus glucosensing neurons. Diabetes 53, 549–559 (2004)
Aponte, Y., Atasoy, D. & Sternson, S. M. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nature Neurosci. 14, 351–355 (2011)
Anthony, T. E. et al. Control of stress-induced persistent anxiety by an extra-amygdala septohypothalamic circuit. Cell 156, 522–536 (2014)
Nordlie, R. C., Foster, J. D. & Lange, A. J. Regulation of glucose production by the liver. Annu. Rev. Nutr. 19, 379–406 (1999)
Bito, H., Deisseroth, K. & Tsien, R. W. CREB phosphorylation and dephosphorylation: a Ca2+- and stimulus duration-dependent switch for hippocampal gene expression. Cell 87, 1203–1214 (1996)
Ghosh, A., Ginty, D. D., Bading, H. & Greenberg, M. E. Calcium regulation of gene expression in neuronal cells. J. Neurobiol. 25, 294–303 (1994)
Lozano, A. M. & Eltahawy, H. How does DBS work? Suppl. Clin. Neurophysiol. 57, 733–736 (2004)
Kühn, F. J., Knop, G. & Luckhoff, A. The transmembrane segment S6 determines cation versus anion selectivity of TRPM2 and TRPM8. J. Biol. Chem. 282, 27598–27609 (2007)
Landau, B. R. & Lubs, H. A. Animal responses to 2-deoxy-d-glucose administration. Proc. Soc. Exp. Biol. Med. 99, 124–127 (1958)
Kim, T., Moore, D. & Fussenegger, M. Genetically programmed superparamagnetic behavior of mammalian cells. J. Biotechnol. 162, 237–245 (2012)
Rowland, N. E., Bellush, L. L. & Carlton, J. Metabolic and neurochemical correlates of glucoprivic feeding. Brain Res. Bull. 14, 617–624 (1985)
Evans, M. L. et al. Hypothalamic ATP-sensitive K+ channels play a key role in sensing hypoglycemia and triggering counterregulatory epinephrine and glucagon responses. Diabetes 53, 2542–2551 (2004)
Murphy, B. A., Fakira, K. A., Song, Z., Beuve, A. & Routh, V. H. AMP-activated protein kinase and nitric oxide regulate the glucose sensitivity of ventromedial hypothalamic glucose-inhibited neurons. Am. J. Physiol. Cell Physiol. 297, C750–C758 (2009)
Horvath, T. L. et al. Brain uncoupling protein 2: uncoupled neuronal mitochondria predict thermal synapses in homeostatic centers. J. Neurosci. 19, 10417–10427 (1999)
Chen, R., Romero, G., Christiansen, M. G., Mohr, A. & Anikeeva, P. Wireless magnetothermal deep brain stimulation. Science 347, 1477–1480 (2015)
Stanley, S. A. et al. Radio-wave heating of iron oxide nanoparticles can regulate plasma glucose in mice. Science 336, 604–608 (2012)
Armbruster, B. N., Li, X., Pausch, M. H., Herlitze, S. & Roth, B. L. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc. Natl Acad. Sci. USA 104, 5163–5168 (2007)
Alexander, G. M. et al. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron 63, 27–39 (2009)
Nawaratne, V. et al. New insights into the function of M4 muscarinic acetylcholine receptors gained using a novel allosteric modulator and a DREADD (designer receptor exclusively activated by a designer drug). Mol. Pharmacol. 74, 1119–1131 (2008)
Falowski, S. M., Ooi, Y. C. & Bakay, R. A. Long-term evaluation of changes in operative technique and hardware-related complications with deep brain stimulation. Neuromodulation 18, 670–677 (2015)
Caterina, M. J. et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389, 816–824 (1997)
Wu, S. Y. & Chiang, C. M. Expression and purification of epitope-tagged multisubunit protein complexes from mammalian cells. Curr. Protoc. Mol. Biol . Chapter 16, Unit 16.13 (2002)
Nakaya, N., Sultana, A., Lee, H. S. & Tomarev, S. I. Olfactomedin 1 interacts with the Nogo A receptor complex to regulate axon growth. J. Biol. Chem. 287, 37171–37184 (2012)
Acknowledgements
We would like to thank A. North, P. Ariel and K. Thomas for help with confocal imaging, D. Acehan and K. Uryu for performing EM studies and S. Korres for assistance with the manuscript. This work was funded by Howard Hughes Medical Institute, the JPB Foundation, the National Institutes of Health (GM095654 and MH105941) and a Rensselaer Fellowship (to J.S.) under an NIH predoctoral training grant (GM067545). Support for this project was provided by a grant from the Robertson Foundation.
Author information
Authors and Affiliations
Contributions
J.M.F. conceived the project and supervised the studies. S.A.S. and L.K. designed and performed the experiments. K.L. and S.F.S. provided technical assistance. A.N. assisted with optogenetic studies, J.S. assisted with magnet activation studies and X.Y. assisted with cell culture studies. J.D. provided technical advice for in vivo magnet activation studies. J.S.D. provided technical advice. S.A.S., L.K. and J.M.F. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Remote neural activation using RF in vivo.
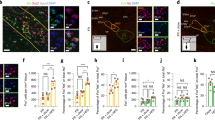
a, Construct design for Ad-FLEX-anti-GFP-TRPV1/GFP-ferritin. CMV, cytomegalovirus promoter. loxN and lox2272 are orthogonal recombination sites. b, Immunostaining for eGFP in GK–Cre/tdTomato mice demonstrates expression of the GFP in glucokinase neurons after VMH injection of Ad-FLEX-anti-GFP-TRPV1/GFP-ferritin. Scale bars, 100 μm; 50 μm in magnification panel. c, Co-localization between eGFP and c-Fos after RF treatment of Nestin–Cre (upper panels) or wild-type (middle panels) mice injected with Ad-FLEX-anti-GFP-TRPV1/GFP-ferritin into the striatum (Scale bar, 80 μm) and of GK–Cre mice injected with Ad-FLEX-anti-GFP-TRPV1/GFP-ferritin into the VMH (lower panels). Scale bar, 100 μm. Quantification of GFP (d) and activated-caspase-3 (e) immunostaining in mice following injection of Ad-anti-GFP-TRPV1/GFP-ferritin or Ad-GFP (1 μl) into the striatum of wild-type (WT) mice or injection of Ad-FLEX-anti-GFP-TRPV1/GFP-ferritin into the VMH of GK–Cre mice. In all cases, columns represent mean and error bars indicate s.e.m. Data were analysed by Kruskal–Wallis test with post-hoc Dunn’s correction. n = 4 mice per group.
Extended Data Figure 2 Effects of RF field strength and treatment duration in vivo.
a, Effect of increasing RF field strength on the change in blood glucose and b, the cumulative change in blood glucose in GK–Cre mice with VMH injection of Ad-FLEX-anti-GFP-TRPV1/GFP-ferritin. Data are shown as mean and error bars indicate s.e.m. Data were analysed by two-way ANOVA with Sidak’s multiple comparisons. * or # indicates P < 0.05, ** or ## indicates P < 0.01, *** or ### indicates P < 0.001, **** or #### indicates P < 0.0001 between treated and untreated groups. c, Effect of increasing RF treatment duration on the change in blood glucose and d, the cumulative change in blood glucose in GK–Cre mice with VMH injection of Ad-FLEX-anti-GFP-TRPV1/GFP-ferritin. Data are shown as mean and error bars indicate s.e.m. Data were analysed by two-way ANOVA with Sidak’s multiple comparisons. * or # indicates P < 0.05, ** or ## indicates P < 0.01, *** or ### indicates P < 0.001, **** or #### indicates P < 0.0001 between treated and untreated groups.
Extended Data Figure 3 Neural activation in GK–Cre and wild-type mice in vivo.
a, RF treatment of GK–Cre mice expressing anti-GFP-TRPV1/GFP-ferritin in the VMH significantly increases blood glucose compared to no RF treatment (n = 13). Data points indicate mean and error bars indicate s.e.m. Data were analysed by two-way ANOVA with Sidak’s multiple comparisons. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 between treated and untreated groups. b, Effects of RF treatment of wild-type mice injected with anti-GFP–TRPV1/GFP–ferritin in the VMH on change in blood glucose with time (n = 10). Data points indicate mean and error bars indicate s.e.m. Data were analysed by two-way ANOVA with Sidak’s multiple comparisons. c, Effects of RF treatment of wild-type mice injected with anti-GFP–TRPV1/GFP–ferritin in the VMH on blood glucose with time (n = 10). Data points indicate mean and error bars indicate s.e.m. Data were analysed by two-way ANOVA with Sidak’s multiple comparisons. d, Effect of RF treatment on cumulative change in blood glucose over the course of the study in wild-type mice with VMH injection of anti-GFP–TRPV1/GFP–ferritin (n = 10). Columns represent mean and error bars indicate s.e.m. Data were analysed by two-tailed, paired Student’s t-test.
Extended Data Figure 4 Remote neural activation of N38 cells expressing anti-GFP–TRPV1/GFP–ferritin in vitro.
a, Calcium imaging of RF-treated N38 cells expressing anti-GFP–TRPV1/GFP–ferritin showing the percentage of cells responding (>20% increase in fluorescence) to no treatment, RF or 2-APB (n = 8, 9 or 2 occasions, respectively), b, the increase in fluorescent signal with RF or 2-APB treatment that is inhibited by Ruthenium red and c, the response time (to reach 20% increase in fluorescence) to RF treatment (n = 38 cells). Data are represented as mean and error bars indicate s.e.m. Data were analysed by Kruskal–Wallis test with Dunn’s multiple comparison test. *P < 0.05 vs. untreated, **P < 0.01 vs. untreated, ***P < 0.001 vs. untreated and ****P < 0.0001 vs. untreated. d, Calcium imaging in stably transfected N38 cells expressing anti-GFP–TRPV1/GFP–ferritin demonstrates a field-strength-dependent increase in the percentage of responding cells (>20% increase in fluorescence) and e, the fluorescent signal in compared to untreated cells; n = 10, 33, 10 and 4 cells, respectively. Data points indicate mean and error bars indicate s.e.m. Data were analysed by two-way ANOVA with Sidak’s multiple comparisons. *P < 0.05, **P < 0.01, ***P < 0.001 ****P < 0.0001 between treated and untreated groups. f, RF treatment of stably transfected N38 cells expressing anti-GFP–TRPV1/GFP–ferritin for 10 s significantly increases the percentage of responding cells and g, the fluorescent signal compared to untreated cells (n = 10 and 11 cells for treated vs. untreated). Data points indicate mean and error bars indicate s.e.m. Data were analysed by unpaired Student’s t-test. *P < 0.05, ***P < 0.001 between treated and untreated groups. h, RF treatment of N38 cells expressing anti-GFP–TRPV1/GFP–ferritin significantly increases pCREB levels and i, relative c-fos (also known as Fos) gene expression (measured by quantitative PCR) and these increases are blocked by Ruthenium red (30 and 100 μM). In all cases, columns represent mean and error bars indicate s.e.m. Data were analysed by one way ANOVA with post-hoc Tukey’s analysis test. Columns marked with **, #, a or & indicate P < 0.01. Each study was repeated on three occasions each with four replicates.
Extended Data Figure 5 RF treatment of N38 cells in vitro.
a, Immunohistochemistry for Flag-tagged ferritin chimaera (red), TRPV1 (blue) and GFP (green) in N38 cells infected with adenovirus expressing anti-GFP–TRPV1/GFP–ferritin. Scale bar represents 20 μm. b, Immunohistochemistry for cell membrane (Alexa-594-conjugated wheat germ agglutinin, red), GFP–ferritin (green) and TRPV1 (blue) in transfected cells showing close proximity of TRPV1 and GFP–ferritin with the cell membrane (upper panels; scale bar, 4 μm) and separate from the cell membrane (lower panels; scale bar, 8 μm). c, Immunoelectron microscopy images from hypothalamic sections taken from GK–Cre mice with unilateral expression of anti-GFP–TRPV1/GFP–ferritin showing immunogold-labelled GFP-tagged ferritin (i; centre and right) from the injected side which are absent on the non-injected side (left, scale bar represents 500 nm and 250 nm for magnification). d, Electron micrograph of iron-loaded ferritin in HEK cells transfected with ferritin construct alone. e, Double immunoelectron microscopy images from stably transfected HEK cells expressing anti-GFP–TRPV1/GFP–ferritin showing co-localization of GFP and TRPV1 at vesicle membranes and at the cell membrane with magnification on the right. Immuno-electron microscopy for GFP and TRPV1 are marked by 5 nm and 12 nm colloidal gold respectively. Scale bar represents 500 nm.
Extended Data Figure 6 RF treatment of N38 cells in vitro.
a, RF treatment of N38 cells does not alter pCREB levels. In all cases, columns represent mean and error bars indicate s.e.m. Each study was repeated on three occasions each with four replicates. b, RF treatment significantly increases relative c-fos gene expression. In all cases, columns represent mean and error bars indicate s.e.m. Data were analysed by two-tailed, unpaired Student’s t-test. *P < 0.05. Each study was repeated on three occasions each with four replicates.
Extended Data Figure 7 Neural inhibition in GK–Cre and wild-type mice in vivo.
a, Effect of RF treatment of N38 cells expressing anti-GFP–TRPV1mutant/GFP–ferritin on pCREB levels and c-Fos expression. In all cases, columns represent mean and error bars indicate s.e.m. Data were analysed by two-tailed Mann–Whitney U-test. *P < 0.05. Each study was repeated on three occasions each with four replicates. b, RF treatment of GK–Cre mice expressing anti-GFP–TRPV1mutant/GFP–ferritin in the VMH significantly decreases blood glucose compared to no RF treatment (n = 13). Data points indicate mean and error bars indicate s.e.m. Data were analysed by two-way ANOVA with Sidak’s multiple comparisons. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 between treated and untreated groups. c, RF treatment significantly decreases cumulative changes in blood glucose over the course of the study in GK–Cre mice with VMH expression of anti-GFP–TRPV1mutant/GFP–ferritin (n = 6) compared to wild-type mice with VMH injection of Ad-FLEX-anti-GFP-TRPV1mutant/GFP-ferritin (n = 9) after administration of 2-deoxyglucose to mimic hypoglycaemia. Data are shown as mean and error bars indicate s.e.m. Data were analysed by unpaired Student’s t-test. *P < 0.05. d, Effects of RF treatment of wild-type mice injected with anti-GFP–TRPV1mutant/GFP–ferritin in the VMH on changes in blood glucose with time (n = 8). Data points indicate mean and error bars indicate s.e.m. Data were analysed by two-way ANOVA with Sidak’s multiple comparisons. e, Effects of RF treatment of wild-type mice injected with anti-GFP–TRPV1mutant/GFP–ferritin in the VMH on cumulative changes in blood glucose with time (n = 8). Data points indicate mean and error bars indicate s.e.m. Data were analysed by two-way ANOVA with Sidak’s multiple comparisons. f, Effect of RF treatment on blood glucose over the course of the study in wild-type mice with VMH injection of anti-GFP– TRPV1/GFP–ferritin (n = 8). Data points represent mean and error bars indicate s.e.m. Data were analysed by two-way ANOVA with Sidak’s multiple comparisons.
Extended Data Figure 8 Magnetic field activation and inhibition of N38 cells in vitro.
Electrophysiological recordings of cultured cells. a, Current trace from a whole-cell voltage-clamp recording (−60 mV) showing the inward current induced with TRPV1 agonist (2-APB 200 μM) in a HEK cell expressing anti-GFP–TRPV1/GFP–ferritin (representative of four recordings). b, Current trace from a whole-cell voltage-clamp recording (−60 mV) induced with a magnetic field (5 s) showing the inward current in stably transfected N38 cells expressing anti-GFP–TRPV1/GFP–ferritin (representative of 15 recordings). c, Bar chart summary of mean peak current induced by TRPV1 agonist 2-APB (200 nM, n = 4) and magnet activation in cultured cells (HEK, n = 10; N38, n = 15) expressing anti-GFP–TRPV1/GFP–ferritin. Columns represent mean and error bars indicate s.e.m. Electrophysiological recordings of cultured cells. d, Current trace from a whole-cell voltage-clamp recording (−60 mV) showing the outward current induced with TRPV1 agonist (2-APB 200 μM) in a HEK cell expressing anti-GFP–TRPV1mutant/GFP–ferritin (representative of 19 recordings). e, Current trace from a whole-cell voltage-clamp recording (−60 mV) induced with a magnetic field (5 s) showing the outward current stably transfected N38 cells expressing anti-GFP–TRPV1mutant/GFP–ferritin (representative of 12 recordings). f, Bar chart summary of mean peak current induced by TRPV1 agonist 2-APB (200 nM, n = 19) and magnet activation in cultured cells (HEK, n = 3; N38, n = 12) expressing anti-GFP–TRPV1mutant /GFP–ferritin. Columns represent mean and error bars indicate s.e.m. g, Summary data showing magnet-induced changes in membrane potential and firing rate for GK VMH neurons expressing anti-GFP–TRPV1/GFP–ferritin and h, anti-GFP–TRPV1mutant/GFP–ferritin. Open circles denote values for individual cells and closed circles denote mean values. Error bars denote s.e.m. For neurons expressing anti-GFP–TRPV1/GFP–ferritin mean membrane potential significantly increased from −70.20 ± 7.246 mV to −53.81 ± 5.349 mV (n = 14, P < 0.0001, paired t-test). Mean firing rate significantly increased from 0.7084 ± 0.2311 to 3.063 ± 0.5632 (n = 16, P < 0.002, paired t-test; includes data from 2 cell-attached recordings). For neurons expressing anti-GFP–TRPV1mutant/GFP–ferritin mean membrane potential significantly decreased from −51.2 ± 5.519 mV to −55.93 ± 5.636 mV (n = 6, P = 0.03; Wilcoxon matched pairs). Mean firing rate significantly decreased from 2.868 ± 1.177 to 0.3167 ± 0.2685 (n = 6, P = 0.03; Wilcoxon matched pairs). Current–voltage relationship of 2-APB-activated TRPV1mutant channels shows limited cation permeability and increased chloride permeability. i, Limited conductance of TRPV1mutant channels compared to wild-type when the predominant internal ions are K+ and gluconate. j, Conductance is increased for anti-GFP–TRPV1mutant channels when the predominant internal ions are Cs and Cl (isometrical chloride). k, Calcium imaging in stably transfected N38 cells expressing anti-GFP–TRPV1/GFP–ferritin demonstrates a magnetic field-strength-dependent increase in the percentage of responding cells (>20% increase in fluorescence) (n = 7, 10, 3 imaging studies, respectively) and l, the fluorescent signal compared to untreated cells (n = 11, 48, 12 cells, respectively). The effects of magnet stimulation were blocked by Ruthenium red. Data points indicate mean and error bars indicate s.e.m. Data were analysed by two-way ANOVA with Sidak’s multiple comparisons. *P < 0.05, **P < 0.01, ***P < 0.001 ****P < 0.0001 between treated and untreated groups. m, Histogram representing the response time (to reach 20% increase in fluorescence) in magnet-treated N38 cells expressing anti-GFP–TRPV1/GFP–ferritin (n = 68 cells). n, Treatment of N38 cells expressing anti-GFP–TRPV1mutant/GFP–ferritin with magnet (n = 6 occasions) significantly increased the percentage of responding cells (i) (>10% decrease in chloride indicator, MQAE,fluorescence) compared to untreated cells (n = 4 occasions) and o, the reduction in MQAE signal. Ruthenium red reduced both the percentage of responding cells and the magnitude of the response (n = 2 occasions). In all cases, columns represent mean and error bars indicate s.e.m. Data were analysed by Kruskal–Wallis test with Dunn’s multiple comparison test. Columns marked with **P < 0.01 vs. untreated, columns marked with ****P < 0.001 vs. untreated.
Extended Data Figure 9 Non-invasive neural activation in vivo using a static magnetic field.
a, The effect of moderate (0.2–0.5 T) magnetic field strength on food intake in GK–Cre mice expressing anti-GFP–TRPV1/GFP–ferritin in the VMH (n = 5). Columns represent mean and error bars indicate s.e.m. b, Schema of the crossover protocol used to examine the effect of neural activation with a static magnetic field on blood glucose. c, Magnetic field treatment of GK–Cre mice following VMH injection of anti-GFP–TRPV1/GFP–ferritin significantly increases blood glucose compared to no magnet treatment (n = 6). Data points indicate mean and error bars indicate s.e.m. Data were analysed by two-way ANOVA with Sidak’s multiple comparisons. d, Magnet treatment significantly increases cumulative change in blood glucose over the course of the study in GK–Cre mice with VMH injection of anti-GFP–TRPV1/GFP–ferritin (n = 6) compared to the same mice without magnet treatment. In all cases, columns represent mean and error bars indicate s.e.m. Data were analysed by Wilcoxon matched pairs signed rank test. *P < 0.05. e, Effects of static magnetic field treatment of wild-type mice with VMH injection of anti-GFP–TRPV1/GFP–ferritin on changes in blood glucose with time mice injected with anti-GFP–TRPV1/GFP–ferritin in the VMH on food intake (n = 6). Data points indicate mean and error bars indicate s.e.m. Data were analysed by two-way ANOVA with Sidak’s multiple comparisons.
Extended Data Figure 10 Baseline characteristics in mice expressing anti-GFP–TRPV1/GFP–ferritin or anti-GFP–TRPV1mutant/GFP–ferritin.
a, Non-fasting blood glucose did not differ significantly between wild-type, GK–Cre and GK–Cre mice injected with anti-GFP–TRPV1/GFP–ferritin or anti-GFP–TRPV1mutant/GFP–ferritin (n = 10, 8, 8 and 5, respectively). Columns represent mean and error bars indicate s.e.m. b, Food intake following a 4 h fast did not differ significantly between wild-type, GK–Cre and GK–Cre mice injected with anti-GFP–TRPV1/GFP–ferritin or anti-GFP–TRPV1mutant/GFP–ferritin (n = 6, 4, 6 and 13 respectively). *P < 0.05. Columns represent mean and error bars indicate s.e.m.
Rights and permissions
About this article
Cite this article
Stanley, S., Kelly, L., Latcha, K. et al. Bidirectional electromagnetic control of the hypothalamus regulates feeding and metabolism. Nature 531, 647–650 (2016). https://doi.org/10.1038/nature17183
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature17183
This article is cited by
-
Magneto-mechanical effect of magnetic microhydrogel for improvement of magnetic neuro-stimulation
Nano Research (2023)
-
Nanoscale thermal effect of ferrimagnetic vortex-domain iron oxide nanorings
Journal of Materials Science (2023)
-
The ventromedial hypothalamic nucleus: watchdog of whole-body glucose homeostasis
Cell & Bioscience (2022)
-
TRPC5 deletion in the central amygdala antagonizes high-fat diet-induced obesity by increasing sympathetic innervation
International Journal of Obesity (2022)
-
Modulating cell signalling in vivo with magnetic nanotransducers
Nature Reviews Methods Primers (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.