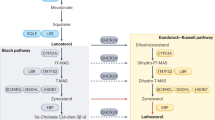
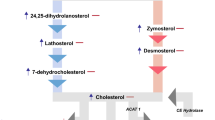
Abstract
The pathogenic event common to all forms of Alzheimer's disease is the abnormal accumulation of the amyloid β-peptide (Aβ). Here we provide strong evidence that intracellular cholesterol compartmentation modulates the generation of Aβ. Using genetic, biochemical and metabolic approaches, we found that cholesteryl-ester levels are directly correlated with Aβ production. Acyl-coenzyme A:cholesterol acyltransferase (ACAT), the enzyme that catalyses the formation of cholesteryl esters, modulates the generation of Aβ through the tight control of the equilibrium between free cholesterol and cholesteryl esters. We also show that pharmacological inhibitors of ACAT, developed for the treatment of atherosclerosis, are potent modulators of Aβ generation, indicating their potential for use in the treatment of Alzheimer's disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Selkoe, D. J. Translating cell biology into therapeutic advances in Alzheimer's disease. Nature 399, A23–A31 (1999).
De Strooper, B. & Annaert, W. Proteolytic processing and cell biological functions of the amyloid precursor protein. J. Cell Sci. 113, 1857–1870 (2000).
Tanzi, R. E. A genetic dichotomy model for the inheritance of Alzheimer's disease and common age-related disorders. J. Clin. Invest. 104, 1175–1179 (1999).
Jarvik, G. P. et al. Interaction of apolipoprotein E genotype, total cholesterol level, and sex in prediction of Alzheimer disease in a case-control study. Neurology 45, 1092–1096 (1995).
Koudinov, A. R., Berezov, T. T. & Koudinova, N. V. Alzheimer's amyloid β and lipid metabolism: a missing link? FASEB J. 12, 1097–1099 (1998).
Lee, S. J. et al. A detergent-insoluble membrane compartment contains Aβ in vivo. Nature Med. 4, 730–734 (1998).
Refolo, L. M., Wittenberg, I. S., Friedrich V. L Jr. & Robakis, N. K. The Alzheimer amyloid precursor is associated with the detergent-insoluble cytoskeleton. J. Neurosci. 11, 3888–3897 (1991).
Parkin, E. T., Turner, A. J. & Hooper, N. M. Amyloid precursor protein, although partially detergent-insoluble in mouse cerebral cortex, behaves as an atypical lipid raft protein. Biochem. J. 344, 23–30 (1999).
Mizuno, T., Haass, C., Michikawa, M. & Yanagisawa, K. Cholesterol-dependent generation of a unique amyloid β-protein from apically missorted amyloid precursor protein in MDCK cells. Biochim. Biophys. Acta 1373, 119–130 (1998).
Bodovitz, S. & Klein, W. L. L. Cholesterol modulates α-secretase cleavage of amyloid precursor protein. J. Biol. Chem. 271, 4436–4440 (1996).
Simons, M. et al. Cholesterol depletion inhibits the generation of β-amyloid in hippocampal neurons. Proc. Natl Acad. Sci. USA 95, 6460–6464 (1998).
Frears, E. R., Stephens, D. J., Walters, C. E., Davies, H. & Austen, B. M. The role of cholesterol in the biosynthesis of β-amyloid. Neuroreport 10, 1699–1705 (1999).
Howland, D. S. et al. Modulation of secreted β-amyloid precursor protein and amyloid β-peptide in brain by cholesterol. J. Biol. Chem. 273, 16576–16582 (1998).
Refolo, L. M. et al. Hypercholesterolemia accelerates the Alzheimer's amyloid pathology in a transgenic mouse model. Neurobiol. Dis. 7, 321–331 (2000).
Brown, M. S., Ho, Y. K. & Goldstein, J. L. The cholesteryl ester cycle in macrophage foam cells. Continual hydrolysis and re-esterification of cytoplasmic cholesteryl esters. J. Biol. Chem. 255, 9344–9352 (1980).
Chang, T. Y., Chang, C. C. & Cheng, D. Acyl-coenzyme A:cholesterol acyltransferase. Annu. Rev. Biochem. 66, 613–638 (1997).
Brown, M. S. & Goldstein, J. L. A proteolytic pathway that controls the cholesterol content of membranes, cells, and blood. Proc. Natl Acad. Sci. USA 96, 11041–11048 (1999).
Simons, K. & Ikonen, E. Functional rafts in cell membranes. Nature 387, 569–572 (1997).
Brown, D. A. & London, E. Functions of lipid rafts in biological membranes. Annu. Rev. Cell. Dev. Biol. 14, 111–136 (1998).
Incardona, J. P. & Eaton, S. Cholesterol in signal transduction. Curr. Opin. Cell Biol. 12, 193–203 (2000).
Blanchette-Mackie, E. J. Intracellular cholesterol trafficking: role of the NPC1 protein. Biochim. Biophys. Acta 1486, 171–183 (2000).
Chang, T. Y. et al. Chinese hamster ovary cell mutants affecting cholesterol metabolism. Curr. Opin. Lipidol. 8, 65–71 (1997).
Reinhart, M. P. Intracellular sterol trafficking. Experientia 46, 599–611 (1990).
Ross, S. L. et al. Amyloid precursor protein processing in sterol regulatory element- binding protein site 2 protease-deficient Chinese hamster ovary cells. J. Biol. Chem. 273, 15309–15312 (1998).
Tomita, T., Chang, T. Y., Kodama, T. & Iwatsubo, T. βAPP γ-secretase and SREBP site 2 protease are two different enzymes. Neuroreport 9, 911–913 (1998).
Chang, C. C. et al. Immunological quantitation and localization of ACAT-1 and ACAT-2 in human liver and small intestine. J. Biol. Chem. 275, 28083–28092 (2000).
Irie, T. et al. Cyclodextrin-induced hemolysis and shape changes of human erythrocytes in vitro. J. Pharmacobiodyn. 5, 741–744 (1982).
Hansen, G. H., Niels-Christiansen, L. L., Thorsen, E., Immerdal, L. & Danielsen, E. M. Cholesterol depletion of enterocytes. Effect on the Golgi complex and apical membrane trafficking. J. Biol. Chem. 275, 5136–5142 (2000).
Rogers, M. J. Statins: lower lipids and better bones? Nature Med. 6, 21–23 (2000).
Wang, I. K., Lin-Shiau, S. Y. & Lin, J. K. Induction of apoptosis by lovastatin through activation of caspase-3 and DNase II in leukaemia HL-60 cells. Pharmacol. Toxicol. 86, 83–91 (2000).
Simons, M. Molecular multitasking: statins lead to more arteries, less plaque. Nature Med. 6, 965–966 (2000).
Yu, G. et al. Nicastrin modulates presenilin-mediated Notch/Glp-1 signal transduction and βAPP processing. Nature 407, 48–54 (2000).
Chan, Y -M & Jan, Y. N. Presenilins, processing of β-amyloid precursor protein, and Notch signaling. Neuron 23, 201–204 (1999).
Brasaemle, D. L., Barber, T., Kimmel, A. R. & Londos, C. Post-translational regulation of perilipin expression. Stabilization by stored intracellular neutral lipids. J. Biol. Chem. 272, 9378–9387 (1997).
Dixon, J. L. & Ginsberg, H. N. Regulation of hepatic secretion of apolipoprotein B-containing lipoproteins: information obtained from cultured liver cells. J. Lipid Res. 34, 167–179 (1993).
Johnson, W. J., Phillips, M. C. & Rothblat, G. H. in Subcellular Biochemistry (ed. Bittman, R.) 235–276 (Plenum, New York, 1997).
Oelkers, P., Behari, A., Cromley, D., Billheimer, J. T. & Sturley, S. L. Characterization of two human genes encoding acyl coenzyme A:cholesterol acyltransferase-related enzymes. J. Biol. Chem. 273, 26765–26771 (1998).
Chang, C. C., Huh, H. Y., Cadigan, K. M. & Chang, T. Y. Molecular cloning and functional expression of human acyl-coenzyme A:cholesterol acyltransferase cDNA in mutant Chinese hamster ovary cells. J. Biol. Chem. 268, 20747–20755 (1993).
Meiner, V. L. et al. Disruption of the acyl-CoA:cholesterol acyltransferase gene in mice: evidence suggesting multiple cholesterol esterification enzymes in mammals. Proc. Natl Acad. Sci. USA 93, 14041–14046 (1996).
Cases, S. et al. ACAT-2, a second mammalian acyl-CoA:cholesterol acyltransferase. Its cloning, expression, and characterization. J. Biol. Chem. 273, 26755–26764 (1998).
Anderson, R. A. et al. Identification of a form of acyl-CoA:cholesterol acyltransferase specific to liver and intestine in nonhuman primates. J. Biol. Chem. 273, 26747–26754 (1998).
Accad, M. et al. Massive xanthomatosis and altered composition of atherosclerotic lesions in hyperlipidemic mice lacking acyl CoA:cholesterol acyltransferase 1. J. Clin. Invest. 105, 711–719 (2000).
Krause, B. R. et al. In vivo evidence that the lipid-regulating activity of the ACAT inhibitor CI-976 in rats is due to inhibition of both intestinal and liver ACAT. J. Lipid Res. 34, 279–294 (1993).
Sugiyama, Y. et al. TMP-153, a novel ACAT inhibitor, inhibits cholesterol absorption and lowers plasma cholesterol in rats and hamsters. Atherosclerosis 113, 71–78 (1995).
Purchase, T. S. et al. Inhibitors of acyl-CoA:cholesterol acyltransferase: novel trisubstituted ureas as hypocholesterolemic agents. Biorg. Med. Chem. 5, 739–747 (1997).
Murakami, S. et al. ACAT inhibitor HL-004 accelerates the regression of hypercholesterolemia in stroke-prone spontaneously hypertensive rats (SHRSP): stimulation of bile acid production by HL-004. Atherosclerosis 133, 97–104 (1997).
Harris, W. S. et al. Effects of the ACAT inhibitor CL277,082 on cholesterol metabolism in humans. Clin. Pharmacol. Ther. 48, 189–194 (1990).
Natory, K., Okazaki, Y., Nakajima, T., Hirohashi, T. & Aono, S. Mechanism of the inhibition of cholesterol absorption by DL-melinamide: inhibition of cholesterol esterification. Jpn J. Pharmacol. 42, 517–523 (1986).
Blaumueller, C. M., Zagouras, P. & Artavanis-Tsakonas, S. Intracellular cleavage of Notch leads to a heterodimeric receptor on the plasma membrane. Cell 90, 281–291 (1997).
Puglielli, L., Rigotti, A., Greco, A. V., Santos, M. J. & Nervi, F. Sterol carrier protein-2 is involved in cholesterol transfer from the endoplasmic reticulum to the plasma membrane in human fibroblasts. J. Biol. Chem. 270, 18723–18726 (1995).
Acknowledgements
We thank C. C. Y. Chang and J. C. Cruz (Dartmouth Medical School, Hanover, New Hampshire) for the gift of cholesterol-mutant cells and competitive inhibitors of ACAT; A. J. Saunders for the CHO cells stably expressing BACE; and K. M. Lentini for her technical support. This work was supported by the American Health Assistance Foundation and the Neurosciences Education and Research Foundation (D.M.K.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Puglielli, L., Konopka, G., Pack-Chung, E. et al. Acyl-coenzyme A: cholesterol acyltransferase modulates the generation of the amyloid β-peptide. Nat Cell Biol 3, 905–912 (2001). https://doi.org/10.1038/ncb1001-905
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1001-905
This article is cited by
-
Relevance of the endoplasmic reticulum-mitochondria axis in cancer diagnosis and therapy
Experimental & Molecular Medicine (2024)
-
Disrupted myelin lipid metabolism differentiates frontotemporal dementia caused by GRN and C9orf72 gene mutations
Acta Neuropathologica Communications (2023)
-
Cholesterol-dependent amyloid β production: space for multifarious interactions between amyloid precursor protein, secretases, and cholesterol
Cell & Bioscience (2023)
-
More than meets the eye in Parkinson’s disease and other synucleinopathies: from proteinopathy to lipidopathy
Acta Neuropathologica (2023)
-
Phosphodiesterase 5 inhibitor mirodenafil ameliorates Alzheimer-like pathology and symptoms by multimodal actions
Alzheimer's Research & Therapy (2022)