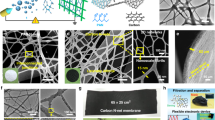
Abstract
One- and two-dimensional carbon nanomaterials are attracting considerable attention because of their extraordinary electrical, mechanical and thermal properties, which could lead to a range of important potential applications. Synthetic processes associated with making these materials can be quite complex and also consume large amounts of energy, so a major challenge is to develop simple and efficient methods to produce them. Here, we present a self-templated, catalyst-free strategy for the synthesis of one-dimensional carbon nanorods by morphology-preserved thermal transformation of rod-shaped metal–organic frameworks. The as-synthesized non-hollow (solid) carbon nanorods can be transformed into two- to six-layered graphene nanoribbons through sonochemical treatment followed by chemical activation. The performance of these metal–organic framework-derived carbon nanorods and graphene nanoribbons in supercapacitor electrodes demonstrates that this synthetic approach can produce functionally useful materials. Moreover, this approach is readily scalable and could be used to produce carbon nanorods and graphene nanoribbons on industrial levels.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Novoselov, K. S. et al.. A roadmap for graphene. Nature 490, 192–200 (2012).
Dai, H. Carbon nanotubes, from synthesis to integration and properties. Acc. Chem. Res. 35, 1035–1044 (2002).
Zhu, Y. et al.. Carbon-based supercapacitors produced by activation of graphene. Science 332, 1537–1541 (2011).
De Jong, K. P. & Geus, J. W. Carbon nanofibers: catalytic synthesis and applications. Catal. Rev. Sci. Eng. 42, 481–510 (2000).
Gadipelli, S. & Guo, Z. X. Graphene-based materials: synthesis and gas sorption, storage and separation. Prog. Mater. Sci. 69, 1–60 (2015).
Arico, A. S., Bruce, P., Scrosati, B., Tarascon, J.-M. & van Schalkwijk, W. Nanostructured materials for advanced energy conversion and storage devices. Nature Mater. 4, 366–377 (2005).
Salunkhe, R. R. et al.. Asymmetric supercapacitors using 3D nanoporous carbon and cobalt oxide electrodes synthesized from a single metal–organic framework. ACS Nano 9, 6288–6296 (2015).
Yang, S., Bachman, R. E., Feng, X. & Müllen, K. Use of organic precursors and graphenes in the controlled synthesis of carbon-containing nanomaterials for energy storage and conversion. Acc. Chem. Res. 46, 116–128 (2013).
Kumar, M. & Ando, Y. Chemical vapor deposition of carbon nanotubes: a review on growth mechanism and mass production. J. Nanosci. Nanotechnol. 10, 3739–3758 (2010).
Kosynkin, D. V. et al.. Longitudinal unzipping of carbon nanotubes to form graphene nanoribbons. Nature 458, 872–876 (2009).
Shinde, D. B., Debgupta, J., Kushwaha, A., Aslam, M. & Pillai, V. K. Electrochemical unzipping of multi-walled carbon nanotubes for facile synthesis of high-quality graphene nanoribbons. J. Am. Chem. Soc. 133, 4168–4171 (2011).
Ma, L., Wang, J. & Ding, F. Recent progress and challenges in graphene nanoribbon synthesis. ChemPhysChem 14, 47–54 (2013).
Terrones, M. et al.. Graphene and graphite nanoribbons: morphology, properties, synthesis, defects and applications. Nano Today 5, 351–372 (2010).
Dutta, S. & Pati, S. K. Novel properties of graphene nanoribbons: a review. J. Mater. Chem. 20, 8207–8223 (2010).
Eddaoudi, M. et al.. Modular chemistry: secondary building units as a basis for the design of highly porous and robust metal−organic carboxylate frameworks. Acc. Chem. Res. 34, 319–330 (2001).
Slater, A. G. & Cooper, A. I. Function-led design of new porous materials. Science 348, aaa8075 (2015).
Yang, S. et al.. Selectivity and direct visualisation of carbon dioxide and sulphur dioxide in a decorated porous host. Nature Chem. 4, 887–894 (2012).
Liu, B., Shioyama, H., Akita, T. & Xu, Q. Metal−organic framework as a template for porous carbon synthesis. J. Am. Chem. Soc. 130, 5390–5391 (2008).
Tang, J. et al.. Thermal conversion of core–shell metal−organic frameworks: a new method for selectively functionalized nanoporous hybrid carbon. J. Am. Chem. Soc. 137, 1572–1580 (2015).
Hao, G. P. et al. Unusual ultra-hydrophilic, porous carbon cuboids for atmospheric-water capture. Angew. Chem. Int. Ed. 54, 1941–1945 (2015).
Das, R., Pachfule, P., Banerjee, R. & Poddar, P. Metal and metal oxide nanoparticle synthesis from metal organic frameworks (MOFs): finding the border of metal and metal oxides. Nanoscale 4, 591–599 (2012).
Xia, W., Mahmood, A., Zou, R. & Xu, Q. Metal–organic frameworks and their derived nanostructures for electrochemical energy storage and conversion. Energy Environ. Sci. 8, 1837–1866 (2015).
Sun, J.-K. & Xu, Q. Functional materials derived from open framework templates/precursors: synthesis and applications. Energy Environ. Sci. 7, 2071–2100 (2014).
Luo, J., Jang, H. D. & Huang, J. Effect of sheet morphology on the scalability of graphene-based ultracapacitors. ACS Nano. 7, 1464–1471 (2013).
Salunkhe, R. R. et al.. Fabrication of symmetric supercapacitors based on MOF-derived nanoporous carbons. J. Mater. Chem. A 2, 19848–19854 (2014).
Xu, X., Cao, R., Jeong, S. & Cho, J. Spindle-like mesoporous α-Fe2O3 anode material prepared from MOF template for high-rate lithium batteries. Nano Lett. 12, 4988–4991 (2012).
Pachfule, P., Biswal, B. P. & Banerjee, R. Control of porosity by using isoreticular zeolitic imidazolate frameworks (IRZIFs) as a template for porous carbon synthesis. Chem. Eur. J. 18, 11399–11408 (2012).
Rowsell, J. L. C. & Yaghi, O. M. Effects of functionalization, catenation, and variation of the metal oxide and organic linking units on the low-pressure hydrogen adsorption properties of metal−organic frameworks. J. Am. Chem. Soc. 128, 1304–1315 (2006).
Ma, J. L., Wong-Foy, A. G. & Matzger, A. J. The role of modulators in controlling layer spacings in a tritopic linker based zirconium 2D microporous coordination polymer. Inorg. Chem. 54, 4591–4593 (2015).
McGuire, C. V. & Forgan, R. S. The surface chemistry of metal–organic frameworks. Chem. Commun. 51, 5199–5217 (2015).
Li, P. et al.. Synthesis of nanocrystals of Zr-based metal–organic frameworks with csq-net: significant enhancement in the degradation of a nerve agent simulant. Chem. Commun. 51, 10925–10928 (2015).
Yu, D., Yazaydin, A. O., Lane, J. R., Dietzel, P. D. C. & Snurr, R. Q. A combined experimental and quantum chemical study of CO2 adsorption in the metal−organic framework CPO-27 with different metals. Chem. Sci. 4, 3544–3556 (2013).
Tranchemontagne, D. J., Hunt, J. R. & Yaghi, O. M. Room temperature synthesis of metal–organic frameworks: MOF-5, MOF-74, MOF-177, MOF-199, and IRMOF-0. Tetrahedron 64, 8553–8557 (2008).
Yue, Y. et al.. Template-free synthesis of hierarchical porous metal–organic frameworks. J. Am. Chem. Soc. 135, 9572–9575 (2013).
Coleman, J. N. Liquid-phase exfoliation of nanotubes and graphene. Adv. Funct. Mater. 19, 3680–3695 (2009).
Jiao, L., Wang, X., Diankov, G., Wang, H. & Dai, H. Facile synthesis of high-quality graphene nanoribbons. Nature Nanotech. 5, 321–325 (2010).
Xu, H. & Suslick, K. S. Sonochemical preparation of functionalized graphenes. J. Am. Chem. Soc. 133, 9148–9151 (2011).
Wang, I. & Kaskel, S. KOH activation of carbon-based materials for energy storage. J. Mater. Chem. 22, 23710–23725 (2012).
Lyon, L. A. Raman spectroscopy. Anal. Chem. 70, 341R–361R (1998).
Jiao, L., Zhang, L., Wang, X., Diankov, G. & Dai, H. Narrow graphene nanoribbons from carbon nanotubes. Nature 458, 877–880 (2009).
Zhang, Z., Sun, Z., Yao, J., Kosynkin, D. V. & Tour, J. M. Transforming carbon nanotube devices into nanoribbon devices. J. Am. Chem. Soc. 131, 13460–13463 (2009).
Rouquerol, F., Rouquerol, J. & Sing, K. Adsorption by Powders and Porous Solids: Principles, Methodology and Applications (Academic, 1999).
Lowell, S., Shields, J. E., Thomas, M. A. & Thommes, M. Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density (Kluwer, 2004).
Rosnes, M. H. et al. Intriguing differences in hydrogen adsorption in CPO-27 materials induced by metal substitution. J. Mater. Chem. A 3, 4827–4839 (2015).
Zhang, W., Wu, Z.-Y., Jiang, H.-L. & Yu, S.-H. Nanowire-directed templating synthesis of metal–organic framework nanofibers and their derived porous doped carbon nanofibers for enhanced electrocatalysis. J. Am. Chem. Soc. 136, 14385–14388 (2014).
Chen, L. et al. One-step solid-state thermolysis of a metal–organic framework: a simple and facile route to large-scale of multiwalled carbon nanotubes. Chem. Commun. 1581–1583 (2008).
Li, J.-S. Nitrogen-doped Fe/Fe3C@graphitic layer/carbon nanotube hybrids derived from MOFs: efficient bifunctional electrocatalysts for ORR and OER. Chem. Commun. 51, 2710–2713 (2015).
Wang, G., Zhang, L. & Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 41, 797–828 (2012).
Gu, W. & Yushin, G. Review of nanostructured carbon materials for electrochemical capacitor applications: advantages and limitations of activated carbon, carbide-derived carbon, zeolite-templated carbon, carbon aerogels, carbon nanotubes, onion-like carbon, and graphene. WIREs Energy Environ. 3, 424–473 (2014).
Miller, J. R., Outlaw, R. A. & Holloway, B. C. Graphene double-layer capacitor with ac line-filtering performance. Science 329, 1637–1639 (2010).
Acknowledgements
The authors thank the reviewers for comments and suggestions, T. Uchida (AIST) and S.K. Soni (RMIT) for microscopic measurements, and Japan Society for the Promotion of Science (JSPS) for financial support (KAKENHI no. 26289379). D.S. and M.M. thank the Australian Research Council for partial support through an ARC Discovery grant (DP 110100082).
Author information
Authors and Affiliations
Contributions
All authors contributed extensively to the work presented in this paper. P.P. and Q.X. conceived the research project. P.P. conducted the experiments and performed the characterizations. D.S. and M.M. recorded the AFM images and performed the conductivity experiments. P.P. and Q.X. wrote the manuscript with the input from the other authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 2188 kb)
Rights and permissions
About this article
Cite this article
Pachfule, P., Shinde, D., Majumder, M. et al. Fabrication of carbon nanorods and graphene nanoribbons from a metal–organic framework. Nature Chem 8, 718–724 (2016). https://doi.org/10.1038/nchem.2515
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2515
This article is cited by
-
Effect of High-Energy Ball Milling in Toluene on the Morphology, Phase Evolution, and Contamination for Some Elemental Powders
Metallurgical and Materials Transactions A (2024)
-
One-dimensionally oriented self-assembly of ordered mesoporous nanofibers featuring tailorable mesophases via kinetic control
Nature Communications (2023)
-
Advanced MOF-based electrode materials for supercapacitors and electrocatalytic oxygen reduction
Nano Research (2023)
-
Template-free synthesis of hollow carbon-based nanostructures from MOFs for rechargeable battery applications
Science China Chemistry (2023)
-
Recent Advances in Metal–Organic Frameworks Based on Electrospinning for Energy Storage
Advanced Fiber Materials (2023)