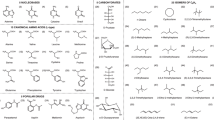
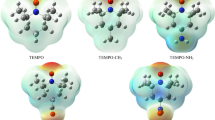
Abstract
The ‘anomeric effect’ is the thermodynamic preference for polar substituents to occupy the axial position in the chair conformation of various heterocycles. The most common explanation given for this effect at present is hyperconjugation from the lone pairs on the ring heteroatom to the antibonding orbital between the anomeric carbon and its linking substituent. Alternatively, the anomeric effect could be explained by intramolecular electrostatic interactions between local dipoles. Few models can provide convincing data for either theory at the quantum-mechanical level. Now, using the extended block-localized wavefunction method, which is the simplest form of valence bond theory, we have evaluated the degree of hyperconjugation in various compounds that display the anomeric effect and have interpreted their conformational preferences in terms of steric, hyperconjugation and dispersion effects. The results provide strong evidence that hyperconjugative interactions are not responsible for the anomeric effect and that it is better interpreted in terms of electrostatic interactions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
28 July 2010
In the version of this Article originally published, Fig. 1 was incorrect. This error has now been corrected in the HTML and PDF versions of the Article.
References
Edward, J. T. Stability of glycosides to acid hydrolysis. Chem. Ind. (Lond.) 1102–1104 (1955).
Lemieux, R. U. & Chü, P. in 133rd National Meeting of the American Chemical Society 31N (American Chemical Society, 1958).
Eliel, E. L. Conformational analysis in heterocyclic systems: recent results and applications. Angew. Chem. Int. Ed. Engl. 11, 739–750 (1972).
Szarek, W. A. & Horton, D. (eds) Anomeric Effect: Origin and Consequences (American Chemical Society, 1979).
Kirby, A. J. Anomeric Effect and Related Stereoelectronic Effects at Oxygen (Springer-Verlag, 1983).
Deslongchamps, P. Stereoelectronic Effects in Organic Chemistry (Elsevier, 1983).
Tvaroska, I. & Bleha, T. Anomeric and exoanomeric effects in carbohydrate chemistry. Adv. Carbohydr. Chem. Biochem. 47, 45–123 (1989).
Thatcher, G. R. (ed.) The Anomeric Effect and Associated Stereoelectronic Effects (American Chemical Society, 1993).
Juaristi, E. & Cuevas, G. The Anomeric Effect (CRC Press, 1995).
Smith, M. B. & March, J. March's Advanced Organic Chemistry (Wiley, 2007).
Anderson, C. B. & Sepp, D. T. Conformation and the anomeric effect in 2-halotetrahydropyrans. J. Org. Chem. 32, 607–611 (1967).
Ha, S. H., Gao, J., Tidor, B., Brady, J. W. & Karplus, M. Solvent effect on the anomeric equilibrium in D-glucose—a free-energy simulation analysis. J. Am. Chem. Soc. 113, 1553–1557 (1991).
Cramer, C. J. Anomeric and reverse anomeric effects in the gas phase and aqueous solution. J. Org. Chem. 57, 7034–7043 (1992).
Fuchs, B., Schleifer, L. & Tartakovsky, E. Structure and conformation of heterocycles .14. probing the anomeric effect—the structural criterion. New J. Chem. 8, 275–278 (1984).
Perrin, C. L., Armstrong, K. B. & Fabian, M. A. The origin of the anomeric effect: conformational analysis of 2-methoxy-1,3-dimethylhexahydropyrimidine. J. Am. Chem. Soc. 116, 715–722 (1994).
Box, V. G. S. The anomeric effect of monosaccharides and their derivatives. Insights from the new QVBMM molecular mechanics force field. Heterocycles 48, 2389–2417 (1998).
Fuchs, B., Ellencweig, A., Tartakovsky, E. & Aped, P. Structure and conformation of heterocycles. 15. Solvent polarity and the anomeric effect. Angew. Chem. Int. Ed. 25, 287–289 (1986).
Juaristi, E. & Cuevas, G. Recent studies of the anomeric effect. Tetrahedron 48, 5019–5087 (1992).
Vila, A. & Mosquera, R. A. Atoms in molecules interpretation of the anomeric effect in the OCO unit. J. Comput. Chem. 28, 1516–1530 (2007).
Salzner, U. & Schleyer, P. v. R. Ab initio examination of anomeric effects in tetrahydropyrans, 1,3-dioxanes and glucose. J. Org. Chem. 59, 2138–2155 (1994).
Reed, A. E., Curtiss, L. A. & Weinhold, F. Intermolecular interactions from a natural bond orbital, donor−acceptor viewpoint. Chem. Rev. 88, 899–926 (1988).
Pophristic, V. & Goodman, L. Hyperconjugation not steric repulsion leads to the staggered structure of ethane. Nature 411, 565–568 (2001).
Bickelhaupt, F. M. & Baerends, E. J. The case for steric repulsion causing the staggered conformation of ethane. Angew. Chem. Int. Ed. 42, 4183–4188 (2003).
Weinhold, F. Rebuttal to the Bickelhaupt–Baerends case for steric repulsion causing the staggered conformation of ethane. Angew. Chem. Int. Ed. 42, 4188–4194 (2003).
Mo, Y. et al. The magnitude of hyperconjugation in ethane: a perspective from ab initio valence bond theory. Angew. Chem. Int. Ed. 43, 1986–1990 (2004).
Mo, Y. & Gao, J. Theoretical analysis of the rotational barrier of ethane. Acc. Chem. Res. 40, 113–119 (2007).
Cooper, D. L. (ed.) Valence Bond Theory (Elsevier, 2002).
Shaik, S. S. & Hiberty, P. C. A Chemist's Guide to Valence Bond Theory (Wiley, 2008).
Wheland, G. W. The Theory of Resonance (John Wiley & Sons, 1944).
Stoll, H., Wagenblast, G. & Preuss, H. On the use of local basis sets for localized molecular orbitals. Theor. Chim. Acta 57, 169–178 (1980).
Mehler, E. L. Self-consistent, nonorthogonal group function approximation for polyatomic systems. II. Analysis of noncovalent interactions. J. Chem. Phys. 74, 6298–6306 (1981).
Gianinetti, E., Raimondi, M. & Tornaghi, E. Modification of the Roothaan equations to exclude BSSE from molecular interaction calculations. Int. J. Quantum Chem. 60, 157–166 (1996).
Mo, Y. & Peyerimhoff, S. D. Theoretical analysis of electronic delocalization. J. Chem. Phys. 109, 1687–1697 (1998).
Mo, Y., Song, L. & Lin, Y. The block-localized wavefunction (BLW) method at the density functional theory (DFT) level. J. Phys. Chem. A 111, 8291–8301 (2007).
Cembran, A., Song, L., Mo, Y. & Gao, J. Block-localized density functional theory (BLDFT), diabatic coupling, and their use in valence bond theory for representing reactive potential energy surfaces. J. Chem. Theory Comput. 5, 2702–2716 (2009).
Mo, Y., Gao, J. & Peyerimhoff, S. D. Energy decomposition analysis of intermolecular interactions using a block-localized wave function approach. J. Chem. Phys. 112, 5530–5538 (2000).
Shaik, S. S., Hiberty, P. C., Lefour, J. M. & Ohanessian, G. Is delocalization a driving force in chemistry? Benzene, allyl radical, cyclobutadiene and their isoelectronic species. J. Am. Chem. Soc. 109, 363–374 (1987).
Hiberty, P. C. & Byrman, C. P. Role of π-electron delocalization in the enhanced acidity of carboxylic acids and enols relative to alcohols. J. Am. Chem. Soc. 117, 9875–9880 (1995).
Lauvergnat, D. & Hiberty, P. C. Role of conjugation in the stabilities and rotational barriers of formamide and thioformamide. An ab initio valence-bond study J. Am. Chem. Soc. 119, 9478–9482 (1997).
Cappel, D., Tullmann, S., Krapp, A. & Frenking, G. Direct estimate of the conjugative and hyperconjugative stabilization in diynes, dienes and related compounds. Angew. Chem. Int. Ed. 44, 3617–3620 (2005).
Linares, M., Braïda, B. & Humbel, S. Lewis-based valence bond scheme: application to the allyl cation J. Phys. Chem. A 110, 2505–2509 (2006).
Wiberg, K. B. & Murcko, M. A. Rotational barriers. 4. Dimethoxymethane—the anomeric effect revisited. J. Am. Chem. Soc. 111, 4821–4828 (1989).
Smith, G. D., Jaffe, R. L. & Yoon, D. Y. Conformational characteristics of dimethoxymethane based upon ab initio electronic structure calculations. J. Phys. Chem. 98, 9072–9077 (1994).
Kneisler, J. R. & Allinger, N. L. Ab initio and density functional theory study of structures and energies for dimethoxymethane as a model for the anomeric effect. J. Comput. Chem. 17, 757–766 (1996).
Bande, A. & Michl, J. Conformational dependence of σ-electron delocalization in linear chains: permethylated oligosilanes. Chem. Eur. J. 15, 8504–8517 (2009).
Eliel, E. L., Hargrave, K. D., Pietrusiewicz, K. M. & Manoharan, M. Conformational analysis. 42. Monosubstituted tetrahydropyrans. J. Am. Chem. Soc. 104, 3635–3643 (1982).
Franck, R. W. A revision of the value for the anomeric effect. Tetrahedron 39, 3251–3252 (1983).
Song, L., Mo, Y., Zhang, Q. & Wu, W. XMVB: a program for ab initio nonorthogonal valence bond computations. J. Comput. Chem. 26, 514–521 (2005).
Schmidt, M. W. et al. General atomic and molecular electronic structure system. J. Comput. Chem. 14, 1347–1363 (1993).
Acknowledgements
This work was supported by the Keck Foundation and Western Michigan University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1325 kb)
Rights and permissions
About this article
Cite this article
Mo, Y. Computational evidence that hyperconjugative interactions are not responsible for the anomeric effect. Nature Chem 2, 666–671 (2010). https://doi.org/10.1038/nchem.721
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.721