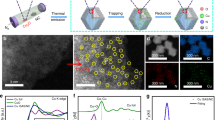
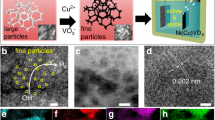
Abstract
The development of high-performance catalysts for the oxygen-evolution reaction (OER) is paramount for cost-effective conversion of renewable electricity to fuels and chemicals. Here we report the significant enhancement of the OER activity of electrodeposited NiOx films resulting from the combined effects of using cerium as a dopant and gold as a metal support. This NiCeOx–Au catalyst delivers high OER activity in alkaline media, and is among the most active OER electrocatalysts yet reported. On the basis of experimental observations and theoretical modelling, we ascribe the activity to a combination of electronic, geometric and support effects, where highly active under-coordinated sites at the oxide support interface are modified by the local chemical binding environment and by doping the host Ni oxide with Ce. The NiCeOx–Au catalyst is further demonstrated in a device context by pairing it with a nickel–molybdenum hydrogen evolution catalyst in a water electrolyser, which delivers 50 mA consistently at 1.5 V over 24 h of continuous operation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bruce, P. G., Freunberger, S. A., Hardwick, L. J. & Tarascon, J.-M. Li–O2 and Li–S batteries with high energy storage. Nature Mater. 11, 19–29 (2012).
Dunn, B., Kamath, H. & Tarascon, J.-M. Electrical energy storage for the grid: a battery of choices. Science 334, 928–935 (2011).
Khaselev, O. & Turner, J. A. A monolithic photovoltaic-photoelectrochemical device for hydrogen production via water splitting. Science 280, 425–427 (1998).
Desmond Ng, J. W., Gorlin, Y., Hatsukade, T. & Jaramillo, T. F. A precious-metal-free regenerative fuel cell for storing renewable electricity. Adv. Energy Mater. 3, 1545–1550 (2013).
Yang, Z. et al. Electrochemical energy storage for green grid. Chem. Rev. 111, 3577–3613 (2011).
Hong, W. T. et al. Toward the rational design of non-precious transition metal oxides for oxygen electrocatalysis. Energy Environ. Sci. 8, 1404–1427 (2015).
Vesborg, P. C. & Jaramillo, T. F. Addressing the terawatt challenge: scalability in the supply of chemical elements for renewable energy. RSC Adv. 2, 7933–7947 (2012).
Haber, J. A. et al. Discovering Ce-rich oxygen evolution catalysts, from high throughput screening to water electrolysis. Energy Environ. Sci. 7, 682–688 (2014).
Subbaraman, R. et al. Trends in activity for the water electrolyser reactions on 3d M(Ni,Co,Fe,Mn) hydr(oxy)oxide catalysts. Nature Mater. 11, 550–557 (2012).
Galán-Mascarós, J. R. Water oxidation at electrodes modified with earth-abundant transition-metal catalysts. ChemElectroChem 2, 37–50 (2015).
Ng, J. W. D., Tang, M. & Jaramillo, T. F. A carbon-free, precious-metal-free, high-performance O2 electrode for regenerative fuel cells and metal–air batteries. Energy Environ. Sci. 7, 2017–2024 (2014).
Suntivich, J., May, K. J., Gasteiger, H. A., Goodenough, J. B. & Shao-Horn, Y. A perovskite oxide optimized for oxygen evolution catalysis from molecular orbital principles. Science 334, 1383–1385 (2011).
Greeley, J. & Markovic, N. M. The road from animal electricity to green energy: combining experiment and theory in electrocatalysis. Energy Environ. Sci. 5, 9246–9256 (2012).
Klingan, K. et al. Water oxidation by amorphous cobalt-based oxides: volume activity and proton transfer to electrolyte bases. ChemSusChem 7, 1301–1310 (2014).
Gerken, J. B., Shaner, S. E., Masse, R. C., Porubsky, N. J. & Stahl, S. S. A survey of diverse earth abundant oxygen evolution electrocatalysts showing enhanced activity from Ni-Fe oxides containing a third metal. Energy Environ. Sci. 7, 2376–2382 (2014).
Gong, M. et al. Nanoscale nickel oxide/nickel heterostructures for active hydrogen evolution electrocatalysis. Nature Commun. 5, 4695 (2014).
Gul, S. et al. Simultaneous detection of electronic structure changes from two elements of a bifunctional catalyst using wavelength-dispersive X-ray emission spectroscopy and in situ electrochemistry. Phys. Chem. Chem. Phys. 17, 8901–8912 (2015).
Lu, X. & Zhao, C. Electrodeposition of hierarchically structured three-dimensional nickel–iron electrodes for efficient oxygen evolution at high current densities. Nature Commun. 6, 6616 (2015).
Smith, R. D. et al. Photochemical route for accessing amorphous metal oxide materials for water oxidation catalysis. Science 340, 60–63 (2013).
Corrigan, D. A. & Bendert, R. M. Effect of coprecipitated metal ions on the electrochemistry of nickel hydroxide thin films: cyclic voltammetry in 1M KOH. J. Electrochem. Soc. 136, 723–728 (1989).
Louie, M. W. & Bell, A. T. An investigation of thin-film Ni–Fe oxide catalysts for the electrochemical evolution of oxygen. J. Am. Chem. Soc. 135, 12329–12337 (2013).
Yeo, B. S. & Bell, A. T. In situ Raman study of nickel oxide and gold-supported nickel oxide catalysts for the electrochemical evolution of oxygen. J. Phys. Chem. C 116, 8394–8400 (2012).
Yeo, B. S. & Bell, A. T. Enhanced activity of gold-supported cobalt oxide for the electrochemical evolution of oxygen. J. Am. Chem. Soc. 133, 5587–5593 (2011).
Gorlin, Y. et al. Understanding interactions between manganese oxide and gold that lead to enhanced activity for electrocatalytic water oxidation. J. Am. Chem. Soc. 136, 4920–4926 (2014).
Frydendal, R. et al. Enhancing activity for the oxygen evolution reaction: the beneficial interaction of gold with manganese and cobalt oxides. ChemCatChem 7, 149–154 (2015).
McCrory, C. C. L. et al. Benchmarking hydrogen evolving reaction and oxygen evolving reaction electrocatalysts for solar water splitting devices. J. Am. Chem. Soc. 137, 4347–4357 (2015).
Burke, M. S., Kast, M. G., Trotochaud, L., Smith, A. M. & Boettcher, S. W. Cobalt–iron (oxy)hydroxide oxygen evolution electrocatalysts: the role of structure and composition on activity, stability, and mechanism. J. Am. Chem. Soc. 137, 3638–3648 (2015).
Trotochaud, L., Young, S. L., Ranney, J. K. & Boettcher, S. W. Nickel–iron oxyhydroxide oxygen-evolution electrocatalysts: the role of intentional and incidental iron incorporation. J. Am. Chem. Soc. 136, 6744–6753 (2014).
Friebel, D. et al. Identification of highly active Fe sites in (Ni,Fe)OOH for electrocatalytic water splitting. J. Am. Chem. Soc. 137, 1305–1313 (2015).
Klaus, S., Cai, Y., Louie, M. W., Trotochaud, L. & Bell, A. T. Effects of Fe electrolyte impurities on Ni(OH)2/NiOOH structure and oxygen evolution activity. J. Phys. Chem. C 119, 7243–7254 (2015).
Zou, S. et al. Fe (Oxy)hydroxide oxygen evolution reaction electrocatalysis: intrinsic activity and the roles of electrical conductivity, substrate, and dissolution. Chem. Mater. 27, 8011–8020 (2015).
Grosvenor, A. P., Biesinger, M. C., Smart, R. S. C. & McIntyre, N. S. New interpretations of XPS spectra of nickel metal and oxides. Surf. Sci. 600, 1771–1779 (2006).
McIntyre, N. S. & Cook, M. G. X-ray photoelectron studies on some oxides and hydroxides of cobalt, nickel, and copper. Anal. Chem. 47, 2208–2213 (1975).
Stefanov, P., Atanasova, G., Stoychev, D. & Marinova, T. Electrochemical deposition of CeO2 on ZrO2 and Al2O3 thin films formed on stainless steel. Surf. Coat. Tech. 180–181, 446–449 (2004).
Yu, X. & Li, G. XPS study of cerium conversion coating on the anodized 2024 aluminum alloy. J. Alloy. Compd. 364, 193–198 (2004).
Batchellor, A. S. & Boettcher, S. W. Pulse-electrodeposited Ni–Fe (oxy)hydroxide oxygen evolution electrocatalysts with high geometric and intrinsic activities at large mass loadings. ACS Catal. 5, 6680–6689 (2015).
Man, I. C. et al. Universality in oxygen evolution electrocatalysis on oxide surfaces. ChemCatChem 3, 1159–1165 (2011).
Nørskov, J. K. et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 108, 17886–17892 (2004).
Li, Y.-F. & Selloni, A. Mechanism and activity of water oxidation on selected surfaces of pure and Fe-doped NiOx . ACS Catal. 4, 1148–1153 (2014).
Strmcnik, D. et al. Improving the hydrogen oxidation reaction rate by promotion of hydroxyl adsorption. Nature Chem. 5, 300–306 (2013).
Vojvodic, A. & Nørskov, J. K. New design paradigm for heterogeneous catalysts. Natl Sci. Rev. 2, 140–149 (2015).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Dudarev, S. L., Botton, G. A., Savrasov, S. Y., Humphreys, C. J. & Sutton, A. P. Electron-energy-loss spectra and the structural stability of nickel oxide: an LSDA+U study. Phys. Rev. B 57, 1505–1509 (1998).
Jain, A. et al. Commentary: the materials project: a materials genome approach to accelerating materials innovation. APL Mater. 1, 011002 (2013).
Fabris, S., de Gironcoli, S., Baroni, S., Vicario, G. & Balducci, G. Taming multiple valency with density functionals: a case study of defective ceria. Phys. Rev. B 71, 041102 (2005).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188–5192 (1976).
Walton, A. S. et al. Interface controlled oxidation states in layered cobalt oxide nanoislands on gold. ACS Nano 9, 2445–2453 (2015).
Rossmeisl, J., Logadottir, A. & Nørskov, J. K. Electrolysis of water on (oxidized) metal surfaces. Chem. Phys. 319, 178–184 (2005).
Acknowledgements
This work was supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences under Award Number DE-SC0008685 and the US Department of Energy Office of Basic Energy Science grant to the SUNCAT Center for Interface Science and Catalysis. Partial support to initiate the project was provided as part of the Center on Nanostructuring for Efficient Energy Conversion (CNEEC) at Stanford University, an Energy Frontier Research Center funded by the US Department of Energy, Office of Science, Office of Basic Energy Sciences under Award Number DE-SC0001060. The authors would like to thank L. Seitz for helpful discussions and R. Kravec for help with XRD measurements. J.W.D.N. acknowledges funding from the Agency of Science, Technology, and Research (A∗STAR), Singapore. M.G.-M. acknowledges funding from the Agency for Administration of University and Research Grants of Catalonia (AGAUR, 2013 BP-A 00464). C.K. acknowledges funding from the Stanford Graduate Fellowship. A.V. acknowledges funding through the SLAC National Accelerator Laboratory LDRD Program.
Author information
Authors and Affiliations
Contributions
J.W.D.N. and T.F.J. conceived and designed the experiments. J.W.D.N., P.C. and C.K. carried out material synthesis. J.W.D.N. and P.C. performed physical and chemical characterization. J.W.D.N., P.C. and C.K. conducted the electrochemical measurements. M.G.-M., M.B. and A.V. formulated, defined and designed the computational part of the paper. M.G.-M. and M.B. performed all the DFT calculations under supervision from A.V. All authors discussed the results and co-wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information.
Supplementary Figures 1–13, Supplementary Table 1, Supplementary Discussion and Supplementary References. (PDF 2277 kb)
Rights and permissions
About this article
Cite this article
Ng, J., García-Melchor, M., Bajdich, M. et al. Gold-supported cerium-doped NiOx catalysts for water oxidation. Nat Energy 1, 16053 (2016). https://doi.org/10.1038/nenergy.2016.53
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nenergy.2016.53
This article is cited by
-
Lamella-heterostructured nanoporous bimetallic iron-cobalt alloy/oxyhydroxide and cerium oxynitride electrodes as stable catalysts for oxygen evolution
Nature Communications (2023)
-
Non-covalent ligand-oxide interaction promotes oxygen evolution
Nature Communications (2023)
-
Electronic optimization and modification of efficient Ir clusters embedded onto Ni–Mo–P for electrocatalytic oxygen evolution reaction
Advanced Composites and Hybrid Materials (2023)
-
Spin-related symmetry breaking induced by half-disordered hybridization in BixEr2-xRu2O7 pyrochlores for acidic oxygen evolution
Nature Communications (2022)
-
Coordination Effect-Promoted Durable Ni(OH)2 for Energy-Saving Hydrogen Evolution from Water/Methanol Co-Electrocatalysis
Nano-Micro Letters (2022)