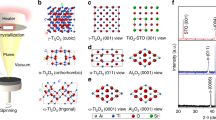
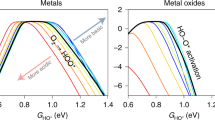
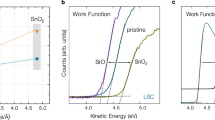
Abstract
Design of cost-effective electrocatalysts with enhanced stability and activity is of paramount importance for the next generation of energy conversion systems, including fuel cells and electrolysers. However, electrocatalytic materials generally improve one of these properties at the expense of the other. Here, using density functional theory calculations and electrochemical surface science measurements, we explore atomic-level features of ultrathin (hydroxy)oxide films on transition metal substrates and demonstrate that these films exhibit both excellent stability and activity for electrocatalytic applications. The films adopt structures with stabilities that significantly exceed bulk Pourbaix limits, including stoichiometries not found in bulk and properties that are tunable by controlling voltage, film composition, and substrate identity. Using nickel (hydroxy)oxide/Pt(111) as an example, we further show how the films enhance activity for hydrogen evolution through a bifunctional effect. The results suggest design principles for this class of electrocatalysts with simultaneously enhanced stability and activity for energy conversion.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Stamenkovic, V. R., Strmcnik, D., Lopes, P. P. & Markovic, N. M. Energy and fuels from electrochemical interfaces. Nat. Mater. 16, 57–69 (2017).
Kodama, K., Jinnouchi, R., Takahashi, N., Murata, H. & Morimoto, Y. Activities and stabilities of Au-modified stepped-Pt single-crystal electrodes as model cathode catalysts in polymer electrolyte fuel cells. J. Am. Chem. Soc. 138, 4194–4200 (2016).
Frydendal, R., Paoli, E. A., Chorkendorff, I., Rossmeisl, J. & Stephens, I. E. L. Toward an active and stable catalyst for oxygen evolution in acidic media: Ti-stabilized MnO2 . Adv. Energy Mater. 5, 1500991 (2015).
Kibsgaard, J., Gorlin, Y., Chen, Z. & Jaramillo, T. F. Meso-structured platinum thin films: active and stable electrocatalysts for the oxygen reduction reaction. J. Am. Chem. Soc. 134, 7758–7765 (2012).
Greeley, J. & Markovic, N. M. The road from animal electricity to green energy: combining experiment and theory in electrocatalysis. Energy Environ. Sci. 5, 9246–9256 (2012).
Paoli, E. A. et al. Oxygen evolution on well-characterized mass-selected Ru and RuO2 nanoparticles. Chem. Sci. 6, 190–196 (2015).
Reier, T., Oezaslan, M. & Strasser, P. Electrocatalytic oxygen evolution reaction (OER) on Ru, Ir, and Pt catalysts: a comparative study of nanoparticles and bulk materials. ACS Catal. 2, 1765–1772 (2012).
Danilovic, N. et al. Using surface segregation to design stable Ru–Ir oxides for the oxygen evolution reaction in acidic environments. Angew. Chem. Int. Ed. 53, 14016–14021 (2014).
Escudero-Escribano, M. et al. Tuning the activity of Pt alloy electrocatalysts by means of the lanthanide contraction. Science 352, 73–76 (2016).
Chang, S. H. et al. Functional links between stability and reactivity of strontium ruthenate single crystals during oxygen evolution. Nat. Commun. 5, 4191 (2014).
Cui, C., Gan, L., Heggen, M., Rudi, S. & Strasser, P. Compositional segregation in shaped Pt alloy nanoparticles and their structural behaviour during electrocatalysis. Nat. Mater. 12, 765–771 (2013).
Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions 2nd edn 644 (National Association of Corrosion Engineers, 1974).
Sheng, W. et al. Non-precious metal electrocatalysts with high activity for hydrogen oxidation reaction in alkaline electrolytes. Energy Environ. Sci. 7, 1719–1724 (2014).
Cui, X., Ren, P., Deng, D., Deng, J. & Bao, X. Single layer graphene encapsulating non-precious metals as high-performance electrocatalysts for water oxidation. Energy Environ. Sci. 9, 123–129 (2016).
Sheng, W. et al. Correlating hydrogen oxidation and evolution activity on platinum at different pH with measured hydrogen binding energy. Nat. Commun. 6, 5848 (2015).
Durst, J. et al. New insights into the electrochemical hydrogen oxidation and evolution reaction mechanism. Energy Environ. Sci. 7, 2255–2260 (2014).
Rizo, R., Herrero, E. & Feliu, J. M. Oxygen reduction reaction on stepped platinum surfaces in alkaline media. Phys. Chem. Chem. Phys. 15, 15416–15425 (2013).
Lee, Y., Suntivich, J., May, K. J., Perry, E. E. & Shao-Horn, Y. Synthesis and activities of rutile IrO2 and RuO2 nanoparticles for oxygen evolution in acid and alkaline solutions. J. Phys. Chem. Lett. 3, 399–404 (2012).
Subbaraman, R. et al. Enhancing hydrogen evolution activity in water splitting by tailoring Li+-Ni(OH)2-Pt interfaces. Science 334, 1256–1260 (2011).
Strmcnik, D. et al. Improving the hydrogen oxidation reaction rate by promotion of hydroxyl adsorption. Nat. Chem. 5, 300–306 (2013).
Subbaraman, R. et al. Trends in activity for the water electrolyser reactions on 3d M(Ni,Co,Fe,Mn) hydr(oxy)oxide catalysts. Nat. Mater. 11, 550–557 (2012).
Danilovic, N. et al. Enhancing the alkaline hydrogen evolution reaction activity through the bifunctionality of Ni(OH)2/metal catalysts. Angew. Chem. Int. Ed. 51, 12495–12498 (2012).
Strmcnik, D., Lopes, P. P., Genorio, B., Stamenkovic, V. R. & Markovic, N. M. Design principles for hydrogen evolution reaction catalyst materials. Nano Energy 29, 29–36 (2016).
Ng, J. W. D. et al. Gold-supported cerium-doped NiOx catalysts for water oxidation. Nat. Energy 1, 16053 (2016).
Görlin, M. et al. Oxygen evolution reaction dynamics, Faradaic charge efficiency, and the active metal redox states of Ni–Fe oxide water splitting electrocatalysts. J. Am. Chem. Soc. 138, 5603–5614 (2016).
Tang, R. et al. Oxygen evolution reaction electrocatalysis on SrIrO3 grown using molecular beam epitaxy. J. Mater. Chem. A 4, 6831–6836 (2016).
Skúlason, E. et al. Catalytic activity of Pt nano-particles for H2 formation. Top. Catal. 57, 273–281 (2014).
Busch, M. et al. Beyond the top of the volcano? —A unified approach to electrocatalytic oxygen reduction and oxygen evolution. Nano Energy 29, 126–135 (2016).
McCrum, I. T. & Janik, M. J. pH and alkali cation effects on the Pt cyclic voltammogram explained using density functional theory. J. Phys. Chem. C 120, 457–471 (2016).
Hörmann, N. G. et al. Some challenges in the first-principles modeling of structures and processes in electrochemical energy storage and transfer. J. Power Sources 275, 531–538 (2015).
Clayborne, A., Chun, H.-J., Rankin, R. B. & Greeley, J. Elucidation of pathways for NO electroreduction on Pt(111) from first principles. Angew. Chem. Int. Ed. 54, 8255–8258 (2015).
Chan, K. & Nørskov, J. K. Potential dependence of electrochemical barriers from ab initio calculations. J. Phys. Chem. Lett. 7, 1686–1690 (2016).
Shaikhutdinov, S. & Freund, H.-J. Ultrathin oxide films on metal supports: structure–reactivity relations. Annu. Rev. Phys. Chem. 63, 619–633 (2012).
Surnev, S., Fortunelli, A. & Netzer, F. P. Structure–property relationship and chemical aspects of oxide–metal hybrid nanostructures. Chem. Rev. 113, 4314–4372 (2013).
Fu, Q., Yang, F. & Bao, X. Interface-confined oxide nanostructures for catalytic oxidation reactions. Acc. Chem. Res. 46, 1692–1701 (2013).
Fu, Q. et al. Interface-confined ferrous centers for catalytic oxidation. Science 328, 1141–1144 (2010).
Merte, L. R. et al. Water-mediated proton hopping on an iron oxide surface. Science 336, 889–893 (2012).
Sun, D. et al. Theoretical study of the role of a metal–cation ensemble at the oxide–metal boundary on CO oxidation. J. Phys. Chem. C 116, 7491–7498 (2012).
Kudernatsch, W. et al. Direct visualization of catalytically active sites at the FeO–Pt(111) interface. ACS Nano 9, 7804–7814 (2015).
Walton, A. S. et al. Interface controlled oxidation states in layered cobalt oxide nanoislands on gold. ACS Nano 9, 2445–2453 (2015).
Merte, L. R. et al. Tuning the reactivity of ultrathin oxides: NO adsorption on monolayer FeO(111). Angew. Chem. Int. Ed. 55, 9267–9271 (2016).
Chen, G. X. et al. Interfacial effects in iron-nickel hydroxide-platinum nanoparticles enhance catalytic oxidation. Science 344, 495–499 (2014).
Yin, H. J. et al. Ultrathin platinum nanowires grown on single-layered nickel hydroxide with high hydrogen evolution activity. Nat. Commun. 6, 6430 (2015).
Wang, L. et al. Optimizing the volmer step by single-layer nickel hydroxide nanosheets in hydrogen evolution reaction of platinum. ACS Catal. 5, 3801–3806 (2015).
Wang, L. et al. Platinum-nickel hydroxide nanocomposites for electrocatalytic reduction of water. Nano Energy 31, 456–461 (2017).
Mansour, A. N. & Melendres, C. A. Analysis of X-ray absorption spectra of some nickel oxycompounds using theoretical standards. J. Phys. Chem. A 102, 65–81 (1998).
Skúlason, E. et al. Modeling the electrochemical hydrogen oxidation and evolution reactions on the basis of density functional theory calculations. J. Phys. Chem. C 114, 18182–18197 (2010).
Zeng, Z. & Greeley, J. Characterization of oxygenated species at water/Pt(111) interfaces from DFT energetics and XPS simulations. Nano Energy 29, 369–377 (2016).
Rossmeisl, J., Chan, K., Skúlason, E., Björketun, M. E. & Tripkovic, V. On the pH dependence of electrochemical proton transfer barriers. Catal. Today 262, 36–40 (2016).
Blochl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Kresse, G. & Furthmuller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Zeng, Z. et al. Towards first principles-based prediction of highly accurate electrochemical Pourbaix diagrams. J. Phys. Chem. C 119, 18177–18187 (2015).
Dudarev, S. L., Botton, G. A., Savrasov, S. Y., Humphreys, C. J. & Sutton, A. P. Electron-energy-loss spectra and the structural stability of nickel oxide: an LSDA+U study. Phys. Rev. B 57, 1505–1509 (1998).
Klimes, J., Bowler, D. R. & Michaelides, A. Van der Waals density functionals applied to solids. Phys. Rev. B 83, 195131 (2011).
Nørskov, J. K. et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 108, 17886–17892 (2004).
Hansen, H. A., Rossmeisl, J. & Norskov, J. K. Surface Pourbaix diagrams and oxygen reduction activity of Pt, Ag and Ni(111) surfaces studied by DFT. Phys. Chem. Chem. Phys. 10, 3722–3730 (2008).
Zeng, Z., Calle-Vallejo, F., Mogensen, M. B. & Rossmeisl, J. Generalized trends in the formation energies of perovskite oxides. Phys. Chem. Chem. Phys. 15, 7526–7533 (2013).
Acknowledgements
This work was supported through a Department of Energy Early Career award through the Office of Science, Office of Basic Energy Sciences, Chemical, Biological, and Geosciences Division under DE-SC0010379. Use of the Center for Nanoscale Materials and the Advanced Photon Source were supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract E-AC02-06CH11357. N.M.M. acknowledges support from the US Department of Energy Office of Science, Basic Energy Sciences, Division of Materials Sciences and Engineering. We acknowledge the computing resources provided on Blues and Fusion, a high-performance computing cluster operated by the Laboratory Computing Resource Center at Argonne National Laboratory, and the National Energy Research Scientific Computing Center (NERSC).
Author information
Authors and Affiliations
Contributions
Z.Z. and J.G. proposed the research direction, designed computations and guided the project. Z.Z. and J.K. performed the computations. K.-C.C. and N.M.M. designed, performed and analysed the experiments. Z.Z. and J.G. analysed the results and drafted the manuscript. All authors discussed the results and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Methods, Supplementary Discussion, Supplementary Tables 1–8, Supplementary Figures 1–31, Supplementary References. (PDF 2148 kb)
Rights and permissions
About this article
Cite this article
Zeng, Z., Chang, KC., Kubal, J. et al. Stabilization of ultrathin (hydroxy)oxide films on transition metal substrates for electrochemical energy conversion. Nat Energy 2, 17070 (2017). https://doi.org/10.1038/nenergy.2017.70
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/nenergy.2017.70
This article is cited by
-
Diversity of platinum-sites at platinum/fullerene interface accelerates alkaline hydrogen evolution
Nature Communications (2023)
-
Direct observation of accelerating hydrogen spillover via surface-lattice-confinement effect
Nature Communications (2023)
-
Adsorbate chemical environment-based machine learning framework for heterogeneous catalysis
Nature Communications (2022)
-
Conversion of Catalytically Inert 2D Bismuth Oxide Nanosheets for Effective Electrochemical Hydrogen Evolution Reaction Catalysis via Oxygen Vacancy Concentration Modulation
Nano-Micro Letters (2022)
-
Orbital coupling of hetero-diatomic nickel-iron site for bifunctional electrocatalysis of CO2 reduction and oxygen evolution
Nature Communications (2021)